From the graph in Figure 11.11, is there any evidence that hydrogen bonding occurs for any elements
Question:
From the graph in Figure 11.11, is there any evidence that hydrogen bonding occurs for any elements other than nitrogen, oxygen, and fluorine? Explain.
Figure 11.11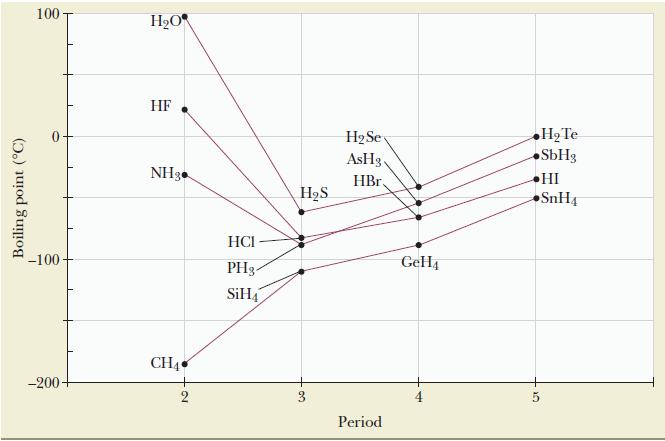
Transcribed Image Text:
Boiling point (C) 100 -100 -200- HO HF NH3 CH4 HCI PH3 SiH4 HS 3 HSe AsHg. HBr. Period GeH4 H Te SbH3 HI 5 SnH4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The graph in Figure 1111 shows the boiling points of various hydrides across different periods of th...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The market research department of a soft drink manufacturer is investigating the effectiveness of a price discount coupon on the purchase of a two-liter beverage product. A sample of 5500 customers...
-
For humans, pregnancy lasts about 280 days. In other species of animals, the length of time from conception to birth varies. Is there any evidence that the gestation period is related to the animal's...
-
Is there any evidence that there is an agency conflict between shareholders and managers when it comes to the payment of dividends? Justify your response.
-
MidWest Amusements is in the process of reviewing 10 proposals for new rides at its theme parks in cities scattered throughout the American heartland. The companys only experienced safety engineer...
-
Why might the revenue and cost figures shown on a standard income statement not be representative of the actual cash inflows and outflows that occurred during a period?
-
A summary of the operations of Quincy Company for the year ended May 31, 2012, is shown below. Required: 1. Determine the net income for the year by preparing an income statement. (There are 8,000...
-
The crop yield for a particular farm in a particular year is typically measured as the amount of the crop produced per acre. For example, cotton is measured in pounds per acre. It has bccn...
-
Suppose you bought a bond with an annual coupon rate of 6.5 percent one year ago for $1,032. The bond sells for $1,020 today. a. Assuming a $1,000 face value, what was your total dollar return on...
-
John's Frozen Pizzas uses FIFO process costing. Selected production and cost data follow for April 2018
-
Explain why high pressures are needed in the industrial process that makes diamond (d = 3.5 g/cm 3 ) from graphite (d = 2.2 g/cm 3 ).
-
State how each of the following properties changes with increasing strength of intermolecular forces. (a) Enthalpy of fusion (b) Melting point (c) Surface tension (d) Viscosity (e) Enthalpy of...
-
The following stream is at 200 psia and 200?F. Determine whether it is a subcooled liquid or a superheated vapor, or whether it is partially vaporized, without making a flashcalculation. Component...
-
P15-29A (similar to) In its annual report, WRM Athletic Supply, Inc. includes the following five-year financial summary: (Click the icon to view the financial summary.) Read the requirements. Current...
-
Toxaway Company is a merchandiser that segments its business into two divisions-Commercial and Residential. The company's accounting Intern was asked to prepare segmented income statements that the...
-
Jimmy Kolop is a manager of a physical therapy department at Bentley Rehab Center. As a unit manager, Jimmy has limitations on exceeding a budget based on a particular item. Bentley Rehab center...
-
Use z scores to compare the given values. Based on sample data, newborn males have weights with a mean of 3233.5 g and a standard deviation of 933.5 g. Newborn females have weights with a mean of...
-
Glycolic acid is produced electrochemically from ethylene glycol under alkaline conditions(naoh). Hydrogen is produced at the cathode, formic acid and oxalic acid are side products Mass balance to...
-
What is the purpose of the reflectron in a time-of-flight mass spectrometer?
-
What are the key dimensions of critical thinking 2. Watch the NBC Learn video on Diet Scams. What types of claims are made in this video Are they valid Elaborate on your responses. Discuss this video...
-
Determine the shape factor for the wide-flange beam. 15 mm 20 mm 200 mm M. 15 mm 200 mm
-
The bar has a thickness of 0.5 in. and is subjected to a moment of 600 lb # ft. Determine the maximum bending stress in the bar. 0.30 in. 6 in. 2 in.
-
The stepped bar has a thickness of 10 mm. Determine the maximum moment that can be applied to its ends if the allowable bending stress is Ï allow = 150 MPa. 90 mm 60 mm 20 mm 7.5 mm 15 mm
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


