From the standard reduction potentials in Table 18.1, find the standard free energy change for the following
Question:
From the standard reduction potentials in Table 18.1, find the standard free energy change for the following reaction:![]()
Table 18.1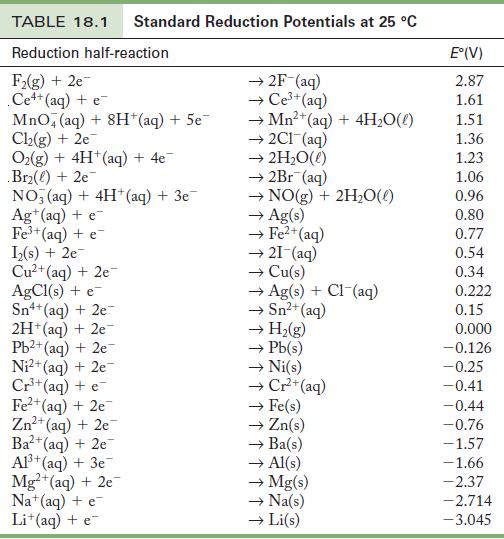
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
AG...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
From the standard reduction potentials listed in Table 19.1 for Zn/Zn2+ and Cu+/Cu2+, calculate ÎG° and the equilibrium constant for the reaction Zn(s) + 2Cu-"(aq)- Zn2+ (aq) + 2Cu+(aq)...
-
Using the standard reduction potentials listed in Table 19.1 and the Handbook of Chemistry and Physics, show that the following reaction is favorable under standard-state conditions: What is the...
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
List three factors that help to determine store image?
-
Consider an opaque, diffuse surface whose spectral reflectivity varies with wavelength as shown. The surface is at 750 K, and irradiation on one side varies with wavelength as shown. The other side...
-
What are the differences between a data entity in structured techniques and an object in object-oriented techniques?
-
(e) Obtain the shrunk mean for each family, and plot the shrunk means against the means obtained when family is specified as a fixed-effect term (the unadjusted means). The point representing one of...
-
The center of gravity G of a 3.5-lb unbalanced tracking wheel is located at a distance r = 0.9 in. from its geometric center B. The radius of the wheel is R = 3 in. and its centroidal radius of...
-
Davidson Industrial bonds have a current market price of $992 and a 5 percent coupon. The bonds pay interest semi-annually on March 1 and September 1. Assume today is January 1. How many months of...
-
Find the equilibrium constant for the following reaction.
-
Most disinfectants kill bacteria by oxidizing them. Which substance is the better oxidant, from the point of standard potentials: Cl 2 or I 2 ?
-
a. Could a linear regression result in residuals 23, -27, 5, 17, -8, 9, and 15? Why or why not? b. Could a linear regression result in residuals 23, -27, 5, 17, -8, -12, and 2 corresponding to x...
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
(a) Why does xenon react with fluorine, whereas neon does not? (b) Using reference sources such as the CRC Handbook of Chemistry and Physics or online sources look up the bond lengths of Xe - F bonds...
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
Two blocks of mass m 1 = 45 kg and m 2 = 12 kg are connected by a mass less string that passes over a pulley as shown in Figure P4.12. The coefficient of static friction between m 1 and the table is...
-
Peel out! The author has a small pickup truck. He finds that it is much easier to burn rubber (i.e., spin the back wheels so that they slip relative to the road surface) when the truck is empty than...
-
A crate is placed on an inclined board as shown in Figure P4.11. One end of the board is hinged so that the angle θ is adjustable. If the coefficient of static friction between the crate...
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year

Study smarter with the SolutionInn App


