Identify the hybrid orbitals used by antimony in SbCl 5 and in SbCl 6 , the
Question:
Identify the hybrid orbitals used by antimony in SbCl5 and in SbCl−6, the ion formed from the reaction of SbCl5 and Cl-. Explain your choices.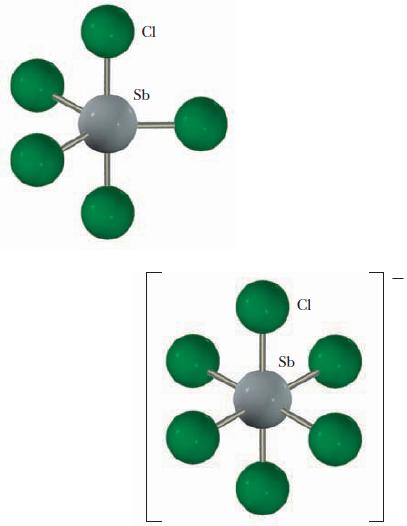
Transcribed Image Text:
CI Sb CI Sb
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
The hybrid orbitals used by antimony Sb in the compounds SbCl and SbCl can be identified by consider...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Identify the hybrid orbitals used by boron in BCl3 and in BCl 4 , the ion formed from the reaction of BCl 3 and Cl - . Explain your choices.
-
Describe the hybrid orbitals used by each carbon atom in the following molecules: a. b. C-C-C-OH
-
Describe the hybrid orbitals used by each nitrogen atom in the following molecules: a. b. H-N-N-H
-
Prudence Corporation manufactures two products: X and Y. The company has 4,000 hours of machine time available and can sell no more than 800 units of product X. Other pertinent data follow. Which of...
-
Sustainable Growth Based on the following information calculates the sustainable growth rate for Hendrix Guitars, Inc.: Profit margin = 6.4% Total asset turnover = 1.70 Total debt ratio = .40 Payout...
-
Why is decision making often described as the essence of a managers job?
-
Traditional forms of coordination can be roughly stereotyped as Japanese centralisation, American formalisation or European socialisation. LO1
-
Ashley attended an in-service education program about the outpatient prospective payment system (OPPS) where she learned that certain Medicare Part B services are paid according to ambulatory payment...
-
gain or loss, before completing the netting procedures
-
Draw the energy-level diagram for the bonding and antibonding molecular orbitals for H 2 . Indicate their relative energies with respect to the 1s atomic orbitals of isolated hydrogen atoms.
-
Compare and contrast the molecular orbital and ionic bonding descriptions of LiF.
-
Let A, B, and C be additive groups and suppose that the sequence is exact. Show that a. j maps B onto C b. i is an isomorphism of A into B c. C is isomorphic to B/i[A] 0 A B C 0
-
Let $N$ be a positive integer. Consider the relation $\circledast$ among pairs of integers $r, s \in \mathbb{Z}$ defined as $r \circledast s$ when $r-s$ is an integer multiple of $N$. Prove that...
-
Draw a circuit diagram for a typical home hair dryer. To which form (or forms) of energy is electric potential energy converted when you use the dryer?
-
Draw a vector field diagram for particles carrying charges \(+2 q\) and \(-q\) separated by a distance \(r\). Comment on the significance of the vector diagram.
-
(a) Show that the Jones matrix of a polarization analyzer set at angle \(\alpha\) to the \(X\)-axis is given by \[ \underline{\mathbf{L}}(\alpha)=\left[\begin{array}{cc} \cos ^{2} \alpha & \sin...
-
Let \(\mathbf{V}(t)\) be a linearly filtered complex-valued, wide-sense stationary random process with sample functions given by \[ \mathbf{v}(t)=\int_{-\infty}^{\infty} \mathbf{h}(t-\tau)...
-
The "rule of three" states that the retention factor for a given solute decreases approximately threefold when the organic phase increases by 10%. In Figure 24-12, tm = 2.7 min. Find k for peak 5 at...
-
Consider the discrete group G of order 8 that has the following Cayley diagram e If we have the sequence of operations: fcagec, which of the options represents the reduction of the sequence to a...
-
Determine the maximum normal stress developed in the bar when it is subjected to a tension of P = 2 kip. 0.125 in. 1.875 in. 1.25 in. r = 0.25 in. 0.75 in.
-
Determine the maximum axial force P that can be applied to the bar. The bar is made from steel and has an allowable stress of Ï allow = 21 ksi. 0.125 in. 1.875 in. 1.25 in. r = 0.25 in. 0.75 in.
-
The A-36 steel plate has a thickness of 12 mm. If Ï allow = 150 MPa, determine the maximum axial load P that it can support. Calculate its elongation, neglecting the effect of the fillets. r= 30...
-
How does budgeting household expenses differ from budgeting business expenses? What are the similarities?
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....

Study smarter with the SolutionInn App


