In the beakers shown below, the colored spheres represent a particular ion, with the dark gray balls
Question:
In the beakers shown below, the colored spheres represent a particular ion, with the dark gray balls representing Pb2+. In one reactant beaker is Pb(NO3)2 and in the other is NaCl. In the product beaker, the organized solid represents an insoluble compound. Write the overall equation, the complete ionic equation, and the net ionic equation.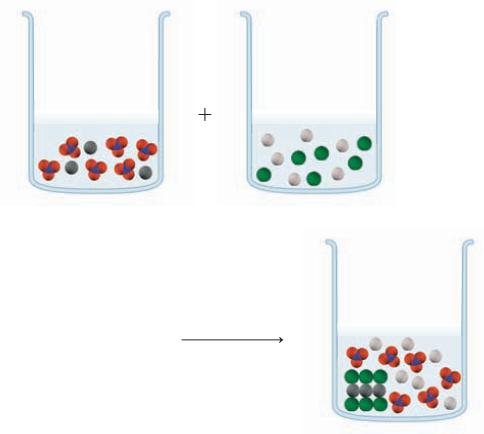
Transcribed Image Text:
+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
overall Pb NO32aq 2NaClaq ...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In the beakers shown below, the colored spheres represent a particular ion, with the dark gray balls representing Ag + . In one reactant beaker is AgNO 3 and in the other is NaBr. In the product...
-
Shown below is the cubic unit cell of an ionic compound. Answer the questions by referring to this structure. Be careful to note that some atoms are hidden by those in front. a. One of the spheres...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Amie, Inc., has 100,000 shares of $2 par value stock outstanding. Prairie Corporation acquired 30,000 of Amie's shares on January 1, 2015, for $120,000 when Amie's net assets had a total fair value...
-
Estimate the minimum frequency of a -ray that causes a deuteron to disintegrate into a proton and a neutron, commenting on any assumptions you make. The masses of the particles are md = 2.0141mu, mp...
-
A partial trial balance of Lindy Corporation at December 31, 2020, follows: Dr. Cr. Supplies $7,700 Salaries and wages payable $5,200 Interest receivable 2,690 Prepaid insurance 113,400 Unearned rent...
-
What do you recommend that Charlies coworkers do to develop more harmonious relationships with him? LO.1
-
The financial statements of The Hershey Company appear in Appendix B, following the financial statements for Tootsie Roll in Appendix A. Instructions (a) Based on the information in the financial...
-
The spending variance for "Other expenses" for October would have been closest to: $400 F $3,625 F $400U $3,625 U The spending variance for "Other expenses" for October would have been closest to:...
-
How much money should be deposited today in an account that earns 4.5% compounded monthly so that it will accumulate to $14,000 in 2 years? Click the icon to view some finance formulas. The amount of...
-
An aqueous sample is known to contain either Pb 2+ or Fe 3+ ions. Treatment of the sample with Na 2 SO 4 produces a precipitate. Use the solubility rules to determine which cation is present.
-
An aqueous sample is known to contain either Mg 2+ or Ba 2+ ions. Treatment of the sample with Na 2 CO 3 produces a precipitate, but treatment with ammonium sulfate does not. Use the solubility rules...
-
Suppose that 2% of convicted felons are in fact innocent. a. If a person is convicted of a felony, what is the probability that he is guilty? b. If two people are convicted of felonies, what is the...
-
Suggest at least 3 touchpoints for each stage of the decision-making process that SEDO can use. Search and find in which of the touchpoints for information search stage suggested by you, can you see...
-
How do modern database management systems address the challenges posed by Big Data, including storage, processing, and analysis of massive volumes of heterogeneous data, while maintaining performance...
-
Describe how the various and sometimes seemingly unrelated topic areas work together toward managing healthcare quality
-
find Fourier series of the following functions (a) f1(x) = sinh(x), (b) f2(x) = cosh(x), (c) f3(x) = x + |x|, (d) f4(x) = x|x|.
-
Explore the realm of database transaction processing, elucidating the nuances of ACID (Atomicity, Consistency, Isolation, Durability) properties and their manifestation in ensuring transactional...
-
What is goodwill ? How does it differ from an intangible asset? Why is a company's internally-generated goodwill not recorded in its accounting records?
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
Use LHpitals rule lim [f(x)/g(x)] x 0 = lim [df(x)dx/dg(x)/dx] x 0 to show that the expression derived for Pf in part b of Example problem 1.1 hane the correct limit as y 0.
-
For each compound below, identify all lone pairs and indicate whether each lone pair is localized or delocalized. Then, use that information to determine the hybridization state and geometry for each...
-
Nicotine is a toxic substance present in tobacco leaves. There are two lone pairs in the structure of nicotine. In general, localized lone pairs are much more reactive than delocalized lone pairs....
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...

Study smarter with the SolutionInn App


