Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen. A two-step mechanism has been
Question:
Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen.![2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/041659664e176f1c1704355041072.jpg)
A two-step mechanism has been proposed. Identify the rate-limiting step.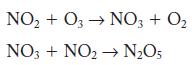
Transcribed Image Text:
2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To identify the ratelimiting step in the twostep reaction mechanism we look at each step and compare ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed to account for the rate law of the decomposition of ozone to O2(g): Apply the steady-state hypothesis to the concentration of atomic oxygen, and derive the...
-
The following mechanism has been proposed for the reaction of NO with H2 to form N2O and H2O: NO(g) + NO(g) N2O2(g) N2O2 + H2(g) N2O(g) + H2O(g) (a) Show that the elementary reactions of the...
-
The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2(g) + ICl(g) HI(g) + HCl(g) HI(g) + ICl(g) I2(g) + HCl(g) (a) Write the balanced equation for the overall...
-
As an employer, what are steps you can take to avoid discrimination litigation?
-
Find Vbd in the circuit infigure. 4 V 6V 12 V
-
What is the current yield for a bond? How are bond prices quoted? How are bonds rated, and why?
-
Understand the role of labor unions in human resource management. LO.1
-
(Default) The Republic of Delinquia has a non-disaster output level of $100 each year. With 10% probability each year, output falls to a disaster level of $80, and the country will feel so much pain...
-
Si una escritura que crea una tenencia en comn NO establece el inters fraccional de cada copropietario, esto: a) es nulo b) cada dueo tiene un inters igual c) debe ser determinada por el voto...
-
Assuming that each reaction is elementary, predict the rate law and molecularity. (a) NO(g) + NO3(g) 2NO(g) (b) O(g) + O3(g) 20(g) (c) (CH3)3CBr(aq) (CH3)3C+ (aq) + Br (aq) (d) 2 HI(g) H(g) + 1(g)
-
The gas-phase reaction of nitrogen monoxide with chlorine proceeds to form nitrosyl chloride. 2NO(g) + Cl(g) 2NOC1(g) rate = k[NO][C1] Evaluate the following proposed mechanism to determine whether...
-
The impact of a drug is a measure of its effect, for example, the reduction in blood pressure, loss of weight, or the duration of a headache. The impact, I, generally depends on the dose, D, given....
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
(a) Which other compounds in Section 13.5 are conjugated dienes? (b) Which other compounds are isolated dienes? (c) Which compound is an isolated enyne?
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
Determine the vertical displacement of joint A. Each bar is made of steel and has a cross-sectional area of 600 mm 2 . Take E = 200 GPa. Use the method of virtual work. B. 2 m AGe 1.5 m 1.5 m 5 kN
-
Use the Muller-Breslau principle to sketch the general shape of the influence line for (a) The moment at A and (b) The shear at B. A
-
Use the Muller-Breslau principle to sketch the general shape of the influence line for (a) The moment at A and (b) The shear at B. TTI A
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...
-
Please note, kindly no handwriting. Q. Suppose a 3 year bond with a 6% coupon rate that was purchased for $760 and had a promised yield of 8%. Suppose that interest rates increased and the price of...

Study smarter with the SolutionInn App


