Nitrogen dioxide decomposes to nitrogen monoxide and oxygen in a second-order reaction. The decrease in concentration of
Question:
Nitrogen dioxide decomposes to nitrogen monoxide and oxygen in a second-order reaction.![]()
The decrease in concentration of NO2 is shown as a function of time in the accompanying figure. Use the data to calculate the rate constant for the reaction.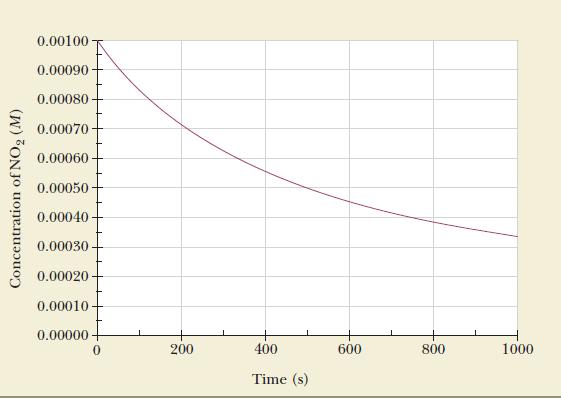
Strategy
Because we are told that the reaction is second order, the graph of 1/[concentration] will be a straight line with a slope equal to the rate constant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





