The equilibrium constant for the decomposition of hydrogen iodide is 0.010 at 307 C. Determine the direction
Question:
The equilibrium constant for the decomposition of hydrogen iodide is 0.010 at 307 °C.![]()
Determine the direction of the reaction if the following amounts (in millimoles, where 1 mmol = 1.0 × 10-3 mol) of each compound are placed in a 1.0-L container.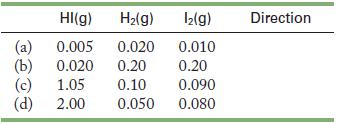
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





