The value of K c for the reaction N(g) + 3H(g) 2NH3(g) is 2.00 at 400 C.
Question:
The value of Kc for the reaction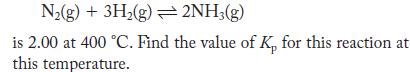
Transcribed Image Text:
N(g) + 3H(g) 2NH3(g) is 2.00 at 400 C. Find the value of K, for this reaction at this temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Solution N2g 3H2g Kp Kc RT ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The value of Kc for the following reaction at 900oC is 0.28. What is Kp at this temperature? CS2(g) +4H2(g)CH4(g) + 2H2S(g)
-
Obtain the value of Kc for the following reaction at 900 K: Use the data given in Problem 14.33. 2502(g) + O2(g) 2SO3(g)
-
Obtain the value of Kc for the following reaction at 500 K: Use the data given in Problem 14.34. CO(g) +2H2(g) CH,OH(g)
-
Marquis Company uses a weighted-average perpetual inventorysystem. August 2, 22 units were purchased at $3 per unit. August 18, 27 units were purchased at $5 per unit. August 29, 24 units were sold....
-
Bill Watts, president of Western Publications, accepts a capital budgeting project proposed by Division X. this is the division in which the president spent his first 10 years with the company. ON...
-
At a press conference in March 2008, Martin Wiederkorn, Volkswagens chief executive, stated that: In the coming years, we will make the VW group the worlds most international carmaker. The days of...
-
When the task is completed, will it be overspent or underspent?
-
Central Michigan Medical Association (CMMA) is planning on hiring a new cardiologist who currently lives in Dallas, Texas. The cardiologist owns home in Dallas. Due to the depressed housing market,...
-
During the financial audit of DIGIT.ALL, the Auditor gathered the following relevant financial data on company performance for the past three years. 2016 2017 2018 2019 sales P220,500.00 P446,250.00...
-
In an experiment, 4.95 mol CO 2 , 0.050 mol CO, and 0.050 mol O 2 are placed in a 5.0-L reaction vessel at 1400 K. Calculate the reaction quotient, Q, for the following reaction: If K c for this...
-
Sulfur dioxide reacts with chlorine when sealed in a reactor at increased temperature. At 227 C, K c = 20.9. Calculate K p at this same temperature. SO(g) + Cl(g) SOCl(g)
-
Study the Minitab R chart for the product and data used in Problem 18.24. Comment on the state of the production process for thisitem. R Chart of Measures 3.0 SL = 2.128 2.0 E0 1.5 R= 1.142 1.0 0.5...
-
a) Discuss whether bike paths can be considered a public good. Now consider a hypothetical town. Suppose that there are three equal-size groups in the economy with the following demand curves: Group...
-
Event services and management can be a lucrative revenue generator. What are the two most important factors in developing a successful event service and management business, whether it is independent...
-
Show how the buying process occurs in the consumer. Review some of the steps in the buying process, stories like: felt need pre-purchase activity purchase decision Post-purchase feelings Explain and...
-
How did Henry Ford set the stage for some of the same problems we still face today in employee relations, especially in manufacturing? 2) If you were a human resources manager, how would you address...
-
What does a DMO risk by not having a positioning theme? Critique the potential of your destination's slogan to effectively differentiate against rivals. you have been asked by a television network to...
-
(a) Draw the condensed structure of the tripeptide Gly-Gly- His. (b) How many different tripeptides can be made from the amino acids glycine and histidine? Give the abbreviations for each of these...
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
A car travels along a straight, level road. The car begins a distance x - 25 m from the origin at t - 0.0 s. At t - 5.0 s, the car is at x - 100 m; at t - 8.0 s, it is at x - 300 m. Find the average...
-
Draw a graph showing the position x as a function of time for an object whose acceleration is (a) Constant and positive, (b) Constant and negative, and (c) Positive and increasing with time. Assume...
-
Draw a possible positiontime graph for an object whose velocity as a function of time is described by (a) Figure P2.28 and (b) Figure P2.29. Figure P2.28 Figure P2.29 v (m/s) HHt (s) 200-40060- 0-...
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


