Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a)
Question:
Use the Ka values in Table 15.6 to calculate the pH of the following solutions.
(a) 0.050 M HI
(b) 0.85 M HF
(c) 0.15 M CH3COOH
(d) 0.017 M C6H5COOH
Table 15.6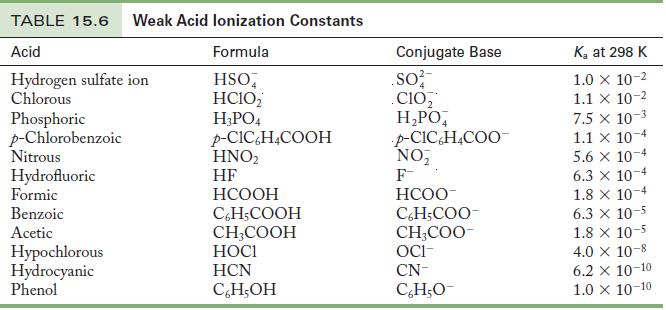
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
General Steps Identify the relevant acid for each solution from the question Locate the correspo...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1 . Which of the following is a device --------------- a .water filter b. mouse c. air conditioning 2. is a unit of physical ---------------- hardware or equipment that provides one or more computing...
-
Don works as a financial adviser in a practice with three other advisers. They each own 25% of the business and while they each look after their own clients, they do share back office, paraplanning...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Evaluate the integral (4e* + 2 In (2))dx.
-
A shell-and-tube heat exchanger with one shell pass and 20 tube passes uses hot water on the tube side to heat oil on the shell side. The single copper tube has inner and outer diameters of 20 and 24...
-
1. Would the name Hallowed receive protection as a trademark or as trade dress? 2. If Trent and Xavier had obtained a business process patent on Hallowed, would the release of Halo 2 infringe on...
-
4. How is the computation of noncontrolling interest share affected by downstream sales of land? By upstream sales of land?
-
The gear forces shown act in planes parallel to the yz plane. The force on gear A is 300 lbf. Consider the bearings at O and B to be simple supports. For a static analysis and a factor of safety of...
-
Example 3-2 Wallington Company has 100 employees, each earning $525.25 a week. OASDI Amount of OASDI tax withheld from each employee's paycheck each week: $32.57 (6.2% $525.25) Total tax withheld...
-
What is the fraction of acid ionized in each acid in Exercise 15.61? Exercise 15.61 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.33 M HNO 2 (b)0.016 M phenol, C 6 H 5 OH (c)0.25 M HF (d) 0.010 M HCOOH Table 15.6
-
On June 30, 2011, Georgia-Atlantic, Inc., leased a warehouse facility from Builders, Inc. The lease agreement calls for Georgia-Atlantic to make semiannual lease payments of $562,907 over a...
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a net ionic equation for the reaction...
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
A block diagram for a 16-bit 2s complement serial subtracter is given here. When St = 1, the registers are loaded and then subtraction occurs. The shift counter, C, produces a signal C15 = 1 after 15...
-
(a) Figure 4-12 shows the block diagram for a 32-bit serial adder with accumulator. The control circuit uses a 5-bit counter, which outputs a signal K = 1 when it is in state 11111. When a start...
-
Write Verilog code for a shift register module that includes a 16-bit shift register, a controller, and a 4-bit down counter. The shifter can shift a variable number of bits depending on a count...
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


