What is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures
Question:
What is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the explosive decomposition of TNT? Use your knowledge of TNT and the chemical equation, particularly the phases, to answer this question.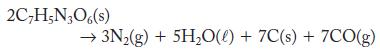
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The provided chemical equation represents the explosive decomposition reaction of trinitrotoluene TNT which is a solid s compound into gaseous nitroge...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the decomposition of phosgene?
-
Reactions between gases in the atmosphere are not at equilibrium, but for a thorough understanding of them we need to study both the rates at which they take place and their behavior under...
-
What is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia?
-
WESTON ENTERPRISES 2019 and 2020 Partial Balance Sheets Assets 2019 2020 Current assets $1,193 $1272 Net fixed assets 5,722 6,023 WESTON ENTERPRISES 2020 Income Statement Sales Costs Depreciation...
-
A large body of non-luminous gas at a temperature of 1200 K has emission bands between 2.5 and 3.5m and between 5 and 8m. The effective emissivity in the first band is 0.8 and in the second 0.6....
-
Define problem recognition. How is this process like translating text from one language into another? What role does probing play in this process?
-
8. Under what circumstances would the direction of intercompany inventory transactions not affect the allocation of unrealized profit?
-
Marston Corporation manufactures pharmaceutical products sold through a network of sales agents in the United States and Canada. The agents are currently paid an 18 percent commission on sales; that...
-
E 9-3 The ledger of Hixson Company at the end of the current year shows Accounts Receivable $120,000, Sales $840,000, and Sales Returns and Allowances $30,000. Instructions (a) If Hixson uses the...
-
The equilibrium constant for the formation of phosgene is measured at two different temperatures. At 506 C, K eq = 1.3; at 530 C, K eq = 0.78. Calculate H and S for this reaction. Under...
-
A 220-ft 3 sample of gas at standard temperature and pressure is compressed into a cylinder, where it exerts pressure of 2000 psi. Calculate the work (inJ) performed when this gas expands...
-
Describe how organizations use assessment of personality type, work behaviors, and job performance to plan employee development.
-
As shown on the attached chart, what is the approximate current 7-year spread premium for Kellogg Bonds? 25 Basis Points 75 Basis Points 200 Basis Points AUS Treasury Actives Curve X-ads Tenor...
-
A pharmaceutical company claims to have invented a new pill to aid weight loss. They claim that people taking these pills will lose more weight than people not taking them. A total of twenty people...
-
Let U = {a, b, c, d, e, f} be the universal set and let A = {a, b, c, d, e, f}. Write the set A. Remember to use correct set notation. Provide your answer below: A=
-
Produce a poster series of three (3) A3 sized posters on creativity in the early years. As a collective the poster series must articulate the importance of aesthetics and creativity for young...
-
Find the second derivative of the function. g(x) = ex In(x) g"(x) = Need Help? Read It
-
The first ionization energy and electron affinity of Ar are both positive values. (a) What is the significance of the positive value in each case? (b) What are the units of electron affinity?
-
Reconsider Prob. 1474. In order to drain the tank faster, a pump is installed near the tank exit as in Fig. P1475. Determine how much pump power input is necessary to establish an average water...
-
A liquid refrigerant (sg = 1.08) is flowing at a weight flow rate of 28.5 N/h. Calculate the volume flow rate and the mass flow rate.
-
Oil with a specific weight of 55.0 lb / ft 3 flows from A to B through the system shown in Fig. 6.35. Calculate the volume flow rate of the oil.
-
Compute the time required to empty the tank shown in Fig. 6.14 if the original depth is 2.68 m. The tank diameter is 3.00 m and the orifice diameter is 150 mm. dh
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


