Write a balanced equation for the reaction pictured below. Nitrogen Oxygen +
Question:
Write a balanced equation for the reaction pictured below.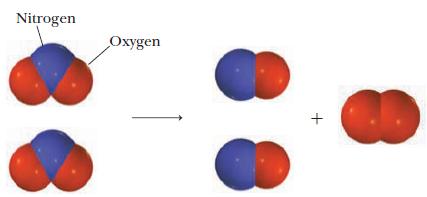
Transcribed Image Text:
Nitrogen Oxygen +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
2N...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced equation for the reaction pictured below. In the diagrams, the red spheres represent oxygen atoms and blue spheres represent nitrogen atoms. Strategy Examine the diagram to determine...
-
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Cesium is added to water. (b) Strontium is added to water. (c) Sodium reacts with oxygen. (d) Calcium reacts...
-
Write a balanced equation for the reaction that occurs in each of the following cases: (a) Ozone decomposes to di oxygen. (b) Xenon reacts with fluorine. (Write three different equations.) (c) Sulfur...
-
public class Class extends ClassA public classB ( ( Time left 0 0 : 1 2 : 0 3 int init = 1 0 ; super ( 4 0 ) ; O a . . The method super is not defined. b . . No values may be passed to super. O c . ....
-
Show that in a good conductor, the skin depth is always much shorter than the wavelength.
-
- Question 12 Not yet answered Project A is expected to generate positive cash flow of $1 million in 10 years while Project Bis expected 15 Marked out of 1.00 22 P Flag question Select one: a....
-
On November 7, 2008, Mura Company borrows $160,000 cash by signing a 90-day, 8% note payable with a face value of $160,000. (1) Compute the accrued interest payable on December 31, 2008, (2) prepare...
-
Kimbrells Furniture Co. sold a new television set and tape player to Charlie ONeil and his wife. Each purchase was on credit, and in each instance, a security agreement was executed. Later on the...
-
Rebecca Mayer is an asset management consultant for institutions and high - net - worth individuals. Mayer meets with Sebastian Capara, the newly appointed Investment Committee chairman for the...
-
Design a solar PV system for a rooftop mounted system for the loads, whose details are given in Table 1. The minimum sunshine hours may be assumed as 5 hrs. Select a 24V DC as the system voltage....
-
Describe the steps needed to write balanced equations.
-
Calculate dollar amounts accurate to the cent and percent amounts to three-figure accuracy. Calculate 1.75% of $350.
-
What are Janes options concerning the ice shortage? LO.1
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
How do individual differences in cognitive styles, such as analytical versus intuitive thinking, impact problem-solving approaches and decision-making processes within teams ?
-
In Russian government, do you think that Russian Military Performance is good in warfare against Ukraine? Explain.
-
Why do you think the competing values framework is important to an organization's effectiveness? Describe the four profiles of the competing values framework. Identify one of the profiles and provide...
-
A mixture of N-acetyl-Leu-Gly, Lys-Gly-Arg, and Lys-Gly-Leu is applied to a sulfonated polystyrene cation-exchange column at a buffer pH of 6.0. Predict the order in which these three peptides will...
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
The normal melting point of H 2 O is 273.15 K, and H fusion = 6010 J mol -1 . Calculate the change in the normal freezing point at 100. and 500. bar compared to that at 1 bar assuming that the...
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
In Equation (8.16), (dP/dT) vaporization was calculated by assuming that V gas m >> V liquid m . In this problem, you will test the validity of this approximation. For water at its normal boiling...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...
-
Horizontal Analysis The comparative accounts payable and long-term debt balances of a company are provided below. Current Year Previous Year Accounts payable $47,286 $63,900 Long-term debt 85,492...
-
On January 1, Year 1, Price Company issued $140,000 of five-year, 7 percent bonds at 97. Interest is payable annually on December 31. The discount is amortized using the straight-line method. Record...

Study smarter with the SolutionInn App


