Write the expression for the equilibrium constant (K c ) for the following: (a) PC15(g) (b) 2NO2(g)
Question:
Write the expression for the equilibrium constant (Kc) for the following: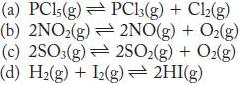
Transcribed Image Text:
(a) PC15(g) (b) 2NO2(g) (c) 2SO3(g) (d) H(g) + 12(g) 2HI(g) PC13(g) + Cl(g) 2NO(g) + O(g) 2SO(g) + O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a K c K PC...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the expression for the equilibrium constant Kc for each of the following equations: a. C(s) CO2(g) 2CO(g) b. FeO(s) +CO(g) Fe(s) CO2(g) c. Na2CO3(s) SO2(g) 02(8)Na SO4(s)CO2(g) d. Pbl2(s) Pb...
-
Write the expression for Kc for the following reactions. In each case indicate whether the reaction is homogeneous or heterogeneous.
-
Write the expression for the thermodynamic equilibrium constant for each of the following reactions. a. b. c. CO(g) 2H2(g) CH,OHg) 2Ag (a CrO4 (aq)Ag CrO4(s) CaCO3(s) +2H (a)Ca2 (aH20CO2(8)
-
Rhenium forms a series of solid oxides: Re2O7 (yellow), ReO3 (red), Re2O5 (blue), and ReO2 (brown). One of them has a crystal structure with the following unit cell: a. How many rhenium atoms (gray...
-
What is supply-chain analysis, and how can it benefit manufactures and retailers?
-
English Garden Landscaping designs and installs landscaping. The landscape designers and office staff use office supplies, while field supplies (rock, bark, etc.) are used in the actual landscaping....
-
What are some ways to implement your recommendation? When youre already big it can be hard to get bigger. Thats why Alibaba is working hard to continue to expand its e-tailing empire. In 1999, Jack...
-
The comparative balance sheet of Navaria Inc. for December 31, 20Y3 and 20Y2, is as follows: The income statement for the year ended December 31, 20Y3, is as follows: Additional data obtained from an...
-
Sep 8 Sold merchandise inventory to Houston Company, S6,300, on account. Terms 1/15, 1/35. Cost of goods, $3,024 Begin by preparing the entry to journalize the sale portion of the transaction. Do not...
-
Temperature influences solubility. Does temperature have the same effect on all substances? Justify your answer.
-
Explain why terms for pure liquids and solids do not appear in the expression for the equilibrium constant.
-
For each of the following lists of premises, derive the indicated conclusion and complete the justification. For double negation, avoid the occurrence of triple tildes.
-
As a job candidate in what way can I apply digital communications to ensure I thrive in the environment of recruiting by using social media for background checks
-
You hold a bond portfolio that consists of (i) a 4-year bond with a face value of $100 that pays an annual coupon of 10%, and (ii) a 2-year bond with a face value of $100 that pays an annual coupon...
-
Draw an original market equilibrium that describes the state of the market before the given scenario occurs. Clearly label both axis, label each a single supply curve and a single demand curve, and...
-
Analyze tools and/or metrics that a leader or manager should use to ensure that they are aligned and working together. Evaluate leadership strategies that could be employed to foster a positive...
-
Mexico has two main government programs that transfer income to rural households. PROCAMPO , which pays a set amount per acre to farmers who grew basic grains in a base year prior to the elimination...
-
Which of these compounds can be a member of an isomer pair? In each case where isomerism is possible, identify the type or types of isomerism. [Sections 24.2, 24.4] CH2 C C-OH --O NHE Cl (b) CH3CH2CH...
-
Write a paper detailing a geographic information system (GIS) of your own design that would utilize data in an original manner.
-
An elite runner can run 1500 m in 3 minutes and 35 s. What is his average speed? Give your answer to two significant figures.
-
A car is traveling on an icy road that is extremely slippery. The driver finds that she is not able to stop or turn the car. Explain this situation in terms of the principle of inertia (Newtons first...
-
A falling baseball has an acceleration of magnitude 9.8 m/s 2 . What is its acceleration in feet per second squared?
-
explain in excel please For a particular product the price per unit is $6. Calculate Revenue if sales in current period is 200 units. Conduct a data analysis, on revenue by changing the number of...
-
Hall Company sells merchandise with a one-year warranty. In the current year, sales consist of 35,000 units. It is estimated that warranty repairs will average $10 per unit sold and 30% of the...
-
Q 4- Crane Corporation, an amusement park, is considering a capital investment in a new exhibit. The exhibit would cost $ 167,270 and have an estimated useful life of 7 years. It can be sold for $...

Study smarter with the SolutionInn App


