Aspirin has the structural formula At body temperature (37C), K a for aspirin equals 3 10
Question:
Aspirin has the structural formula
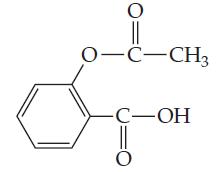
At body temperature (37°C), Ka for aspirin equals 3 × 10-5. If two aspirin tablets, each having a mass of 325 mg, are dissolved in a full stomach whose volume is 1 L and whose pH is 2, what percent of the aspirin is in the form of neutral molecules?
Transcribed Image Text:
0-C-CH3 -С—ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
Body temperature 37 The percent of ionization is The percent of aspirin in neutra...View the full answer

Answered By

Desmond Boakye-Agyemang
I have been teaching and preparing students to write exams for more than seven years and with hands on practicals of chemistry through higher education, teaching has been very easy and students appreciate my efforts and way of teaching.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Related Video
For this experiment, we\'ve compared the freshness of flowers by keeping them in three separate bottles filled with water, aspiring water, and food plant
Students also viewed these Sciences questions
-
When perspiration on the human body absorbs heat, some of the perspiration turns into water vapor. The latent heat of vaporization at body temperature (37 C) is 2.42 106 J/kg. The heat absorbed is...
-
(a) Calculate the percent ionization of a 0.20 M solution of the monoprotic acetylsalicylic acid (aspirin) for which Ka 5 3.0 3 10-4. (b) The pH of gastric juice in the stomach of a certain...
-
One suggested treatment for a person who has suffered a stroke is immersion in an ice-water bath at 0C to lower the body temperature, which prevents damage to the brain. In one set of tests, patients...
-
discusses how a reseller can service both a consumer and an industrial market from the same store location. Provide an example of a retailer and detail the differences in their marketing activities.
-
Refer to the cause-and-effect diagram on page 17. The workers have now noticed that a delay could occur: (a) On the fourth floor at the pharmacy (b) On the third floor at the practitioners' station...
-
Accounting versus Tax Treatment (Appendix-related) Pineco plans to embark on a business acqui sition program to diversify its product lines. The controller is unclear about whether the tax treat ment...
-
EXERCISE 22 Apply Overhead Cost to Jobs LO22 Luthan Company uses a plantwide predetermined overhead rate of $23.40 per direct labor-hour. This predetermined rate was based on a cost formula that...
-
According to the American Lung Association, 90% of adult smokers started smoking before turning 21 years old. Ten smokers 21 years old or older are randomly selected, and the number of smokers who...
-
Jamaica corp. is adding a new assembly line at a cost of $8 million. The firm expects the project to generate cash flows of $2 million, $3 million, $4 million, and $5 million over the next four...
-
One out of 279937 dices is produced with a fault that number "l" is printed on all faces. A person rolls a dice 7 times and observes that all 7 rolls come up with number "1" (a) State the Bayes\'...
-
A sample of 7.5 L of NH 3 gas at 22C and 735 torr is bubbled into a 0.50-L solution of 0.40 M HCl. Assuming that all the NH 3 dissolves and that the volume of the solution remains 0.50 L, calculate...
-
What is the pH at 25 C of water saturated with CO 2 at a partial pressure of 1.10 atm? The Henry's law constant for CO 2 at 25 C is 3.1 10 2 mol/L-atm. The CO 2 is an acidic oxide, reading with H 2...
-
Estimate the percentages in Problems 2228. a. 40% of 93 b. 90% of 8,741
-
An employer has calculated the following amounts for an employee during the last week of June 2021. Gross Wages $1,800.00 Income Taxes $414.00 Canada Pension Plan $94.00 Employment Insurance $28.00...
-
Section Two: CASE ANALYSIS (Marks: 5) Please read the following case and answer the two questions given at the end of the case. Zara's Competitive Advantage Fashion houses such as Armani and Gucci...
-
The activity of carbon in liquid iron-carbon alloys is determined by equilibration of CO/CO2 gas mixtures with the melt. Experimentally at PT = 1 atm, and 1560C (1833 K) the equilibrated gas...
-
Apply knowledge of concepts and theories covered in the course to leader - the leader can either be themselves if they lead a team, someone real and personally known to them (such as a boss or leader...
-
A resistor in a dc circuit R = 1.2 2. The power dissipated P is a second-degree function of the voltage V. Graph P versus V from V = 0.0 V to V = 3.0 V.
-
Sketch the region bounded by the curves. y = 3x, y = x 2
-
C- Consider the following scenario:- A supermarket needs to develop the following software to encourage regular customers. For this, the customer needs to supply his/her residence address, telephone...
-
Vector A starts at point (1, - 1, - 3) and ends at point (2, - 1, 0). Find a unit vector in the direction of A.
-
Given vectors A = x2 y3 z, B = x2 y z3, and C = x4 y2 z2, show that C is perpendicular to both A and B.
-
In Cartesian coordinates, the three corners of a triangle are P1 (0, 4, 4). P2 (4, - 4, 4), and P3 (2, 2, - 4) find the area of the triangle.
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


