Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The activity of carbon in liquid iron-carbon alloys is determined by equilibration of CO/CO2 gas mixtures with the melt. Experimentally at PT = 1
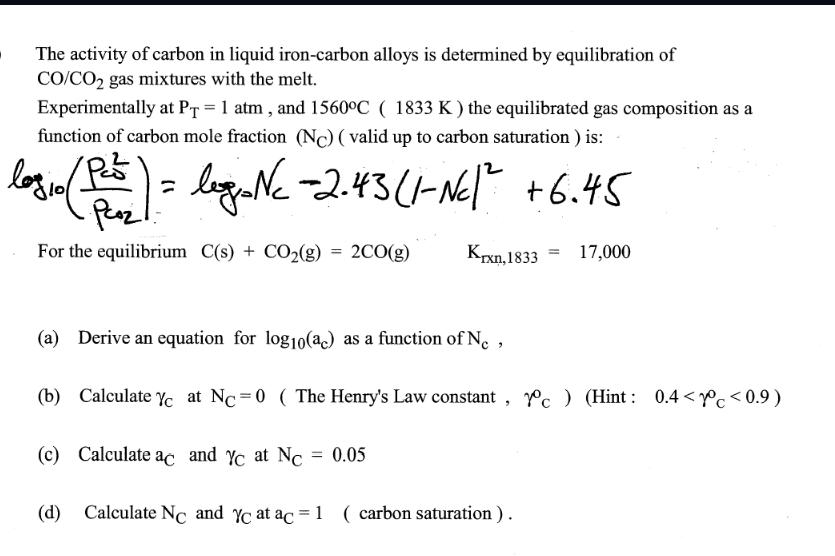
The activity of carbon in liquid iron-carbon alloys is determined by equilibration of CO/CO2 gas mixtures with the melt. Experimentally at PT = 1 atm, and 1560C (1833 K) the equilibrated gas composition as a function of carbon mole fraction (NC) (valid up to carbon saturation) is: log 10 (Pass) = legro NC - 2.43 (1-NC1 +6.45 Poz For the equilibrium C(s) + CO2(g) = 2CO(g) Krxn, 1833 = 17,000 (a) Derive an equation for log10(ac) as a function of NC, (b) Calculate YC at NC = 0 ( The Henry's Law constant, 1c) (Hint: 0.4
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To derive an equation for log10aC as a function of Nc we can use the given equilibrium equation Cs ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


