Question
Use 5 Data tables and 3 figures provided. Acetaminophen is well known pain reliver (it is an active ingredient in brand such as Tylenol). Active
Use 5 Data tables and 3 figures provided.
Acetaminophen is well known pain reliver (it is an active ingredient in brand such as Tylenol).
Active drug is processed to make a D.C. grade and a coated grade of materials. 525 mg of D.C. material has 500 mg Active (formulation I and II); and 566 mg of Coated material has 500 mg of active (formulation III and IV). This relates to 700 mg total weight of tablet. These two materials are used to develop formulation.
The properties are compared to crystalline powder, see the following Data.
| Ingredients | Mean particle sizeμ | Bulk density (g/ml) | Tapped Density g/ml | Angle of repose | |
| Acetaminophen Crystal | >355 | 0.382 | 0.259 | Not done | |
| Acetaminophen D.C. | >45 | 0.679 | 0.530 | 41.63 | |
| Acetaminophen coated | >250 | 0.469 | 0.417 | 39.52 | |
The following Formulations compositions, % W/W, are studied. Brand names of some excipients are also listed. Please do own research about these ingredients and other info.
| Ingredients | Formulation I %w/w | Formulation II% w/w | Formulation III %w/w | Formulation IV %w/w |
| Acetaminophen D.C. | 75 | 75 | - | - |
| Acetaminophen coated | - | - | 80.85 | 80.85 |
| vinylpyrrolidone-vinyl acetate copolymers, Kolidon VA 64 | 4 | 4 | 2 | 2 |
| Croscarmellose Sodium Vivasol© | - | 5 | - | 5 |
| crospovidone Polyplasdone® XL-10 | 4 | - | 4 | - |
| Microcrystalline Cellulose Avicel PH 200 | 12.5 | 11.5 | 8.64 | 7.64 |
| hydroxypropyl cellulose Klucel EXF | 2 | 2 | 2 | 2 |
| PEG 4000 | 1 | 1 | 1 | 1 |
| Mg-stearate | 1 | 1 | 1 | 1 |
| Fumed silica Aerosil 200 | 0.5 | 0.5 | 0.5 | 0.5 |
| % W/W Total | 100.0 % | 100.0 % | 100.0 % | 100.0 % |
| Weight of Tablet mg | 700 | 700 | 700 | 700 |
| Weight of material containing API: mg | 525 | 525 | 566 | 566 |
| Acetaminophen Dose Potency mg | 500 | 500 | 500 | 500 |
Question: Please answer those questions from the data!!!
- Write manufacturing procedure(s) in sufficient detail (not one liner) for 100 kg batch. Score 3
- Provide equipment used in the process, explain equipment capacity, describe operating conditions, including size of tablet for 500 mg dose. Score 3
- Show (Draw) the Compaction profiles, i.e., Pressure vs Time for formulation during the pressing of powder for formulation of ONE formulation you concluded is the best. (If you give two or more formulations, then you will get 0) for entire test).
Explain why did you the formulation is the best? Include several tables with analysis of data (tables and graphs) in your answers (Score 0 for only words). Score 6
| Final Powder mix | Mean particle size μ | Bulk density (g/ml) | Tapped Density g/mL |
| I: Acetaminophen D.C.+Polyplasdone XL-10 | >45 | 0.479 | 0.388 |
| II: Acetaminophen D.C.+Vivasol | >45 | 0.483 | 0.393 |
| III: Acetaminophen coated+Polyplasdone XL-10 | >250 | 0.609 | 0.513 |
| III: Acetaminophen coated+Vivasol | >250 | 0.608 | 0.507 |
Tablet Physical Properties:
| Tablet | Weight (g) | Porosity (%) | Hardness (N) | Friability (%) | |
| 0.709±1.84 | 15.45 | 153.19 | 0.52 | |
| Formula II | 0.705±0.43 | 59.43 | 150.46 | 0.51 | |
| Formula III | 0.702±0.03 | 59.23 | 71.99 | 0.24 | |
| Formula IV | 0.702±0.52 | 59.42 | 65.3 | 0.24 | |
| Brand as Reference | 0.604±0.10 | Not done | 61.3 | 0.91 |
Data: Dissolution at pH 1, 4.5 and 5.8 Formulations: I, II, III and IV.
pH 1.2
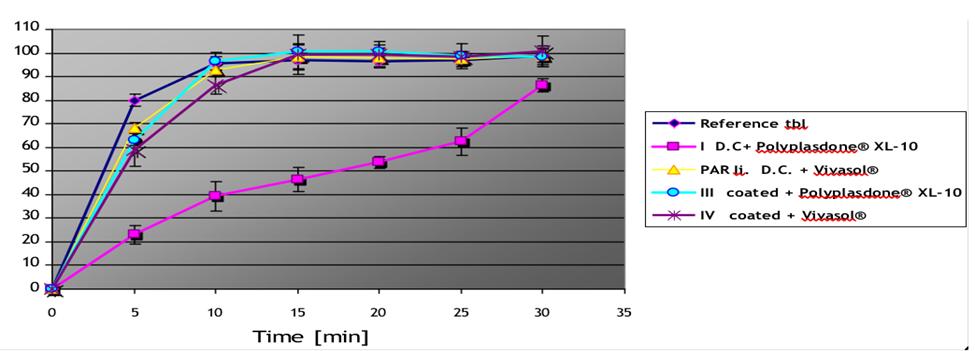
pH; 4.5
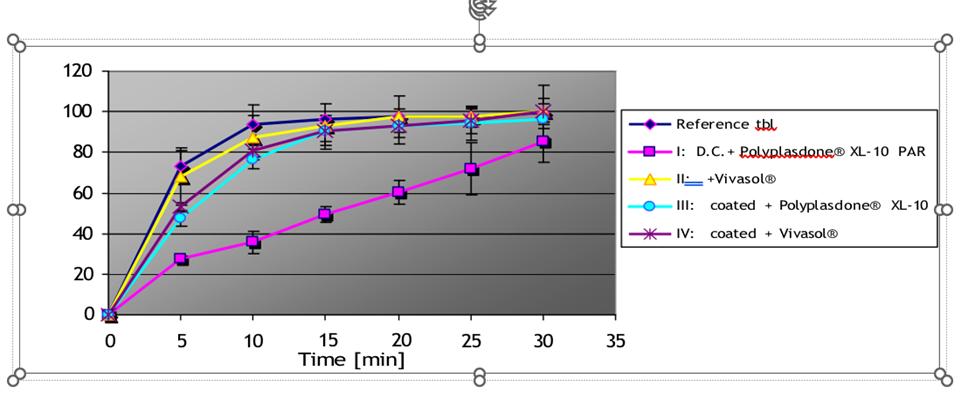
pH 5.8
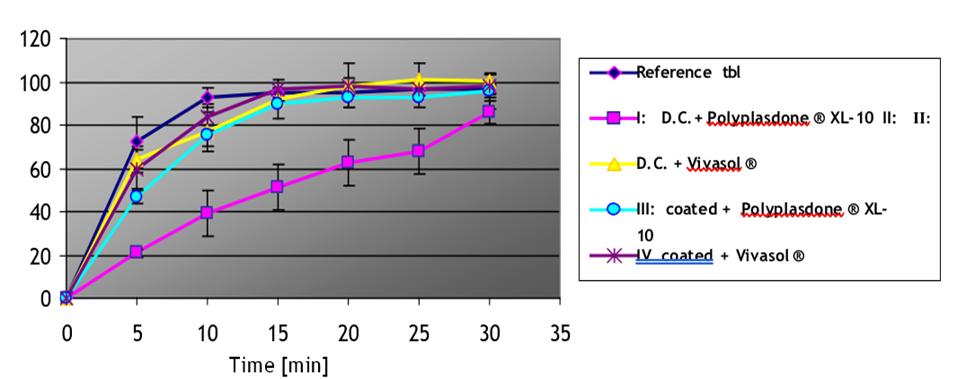
110 100 90 80 70 60 50 40 30 20 10 5 10 15 Time [min] 20 25 30 35 Reference tbl ID.C+ Polyplasdone XL-10 PAR li. D.C. + Vivasol III coated Polyplasdone XL-10 IV coated + Vivasol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


