Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within
Question:
Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within the liquid can be stronger or weaker than the adhesive forces between liquid and surface:
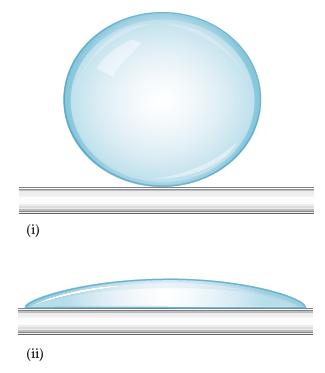
(a) In which of these diagrams, i or ii, do the adhesive forces between surface and liquid exceed the cohesive forces within the liquid?
(b) Which of these diagrams, i or ii, represents what happens when water is on a nonpolar surface?
(c) Which of these diagrams, i or ii, represents what happens when water is on a polar surface?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





