Potassium and hydrogen react to form the ionic compound potassium hydride. (a) Write a balanced equation for
Question:
Potassium and hydrogen react to form the ionic compound potassium hydride.
(a) Write a balanced equation for this reaction.
(b) Use data in Figures 7.10 and 7.12 to determine the energy change in kJ/mol for the following two reactions:
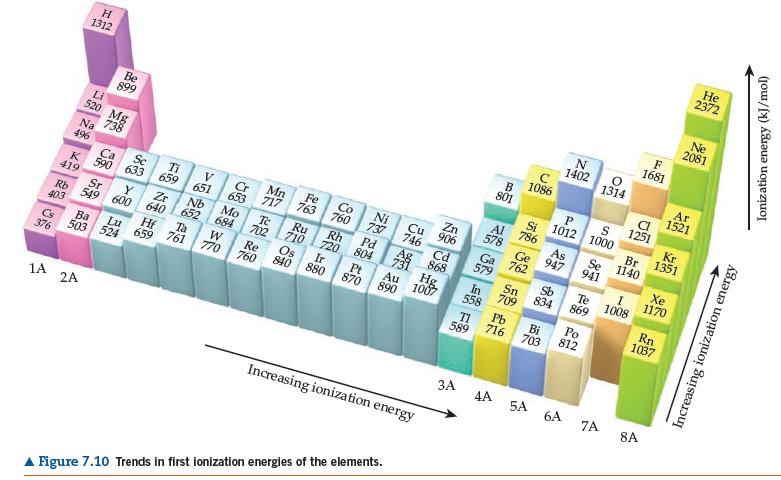
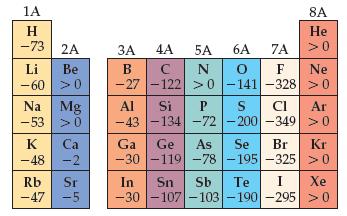
Figures 7.12
K(g) + H(g) → K+(g) + H-(g)
K(g) + H(g) → K-(g) + H+(g)
(c) Based on your calculated energy changes in (b), which of these reactions is energetically more favorable (or less unfavorable)?
(d) Is your answer to (c) consistent with the description of potassium hydride as containing hydride ions?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





