The experimental Bi - I bond length in bismuth triiodide, BiI 3 , is 2.81 . Based
Question:
The experimental Bi - I bond length in bismuth triiodide, BiI3, is 2.81 Å. Based on this value and data in Figure 7.6, predict the atomic radius of Bi.
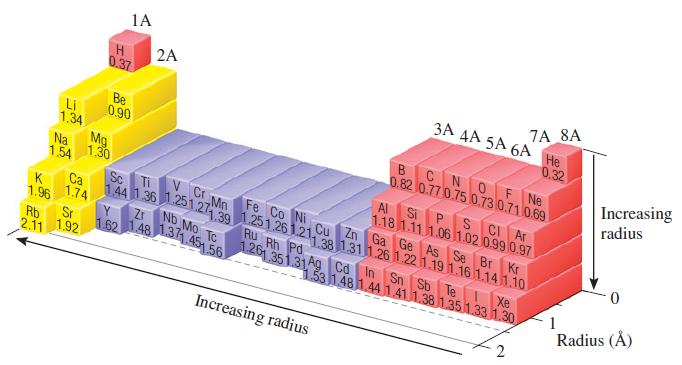
Transcribed Image Text:
1A H 2A 7A 8A 3A AA 5A 6A 0.37 Не 0.32 BCNOF Ne 0.82 0.77 0.75 0.73 0.71 0.69 Be 0.90 Li 1.34 Mg Increasing Na 1.30 1.54 radius Al S CI Ar P. 1.18 1.11 1.06 1.02 0.99 0.97 Cu Zn Ga Ge As Se Br Kr Si V C Mn Sc Ca 144 1 36.251.27 39 1.251.26 1.21,28 1311 261.22 1.19 1.16 1.141.10 1.74 Fe Co Ni K 1.96 Zr No Mo TC Ru Rh Pd Rb 2.11 1.92 Sr 162 1.48 131.45 56 126 351.31A9 Cơ In Sn Sb Te Xe 1.53 148 1.44 1.41 1.38 1.351.33 1.30 Radius (Å) Increasing radius
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The bond distance of 281 angstrom for ...View the full answer

Answered By

MORINKE KUDAOS
I am an educator that has the necessary abilities and expertise owing to extensive interaction with students. I present answers to a variety of problems with step-by-step explanations, a well-thought-out strategy, and an easily understood breakdown. My objective is to teach students more easy methods and comprehension so that they may attain more success.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
(a) The measured Bi - Br bond length in bismuth tribromide, BiBr3, is 2.63 . Based on this value and the data in Figure 7.7, predict the atomic radius of Bi.
-
The triiodide ion (I-3) in which the I atoms are arranged in a straight line is stable, but the corresponding F-3 ion does not exist. Explain.
-
Estimate the As I bond length from the data in Figure 7.6, and compare your value to the experimental As I bond length in arsenic triiodide, AsI 3 , 2.55 . 1A H 2A 7A 8A 3A AA 5A 6A 0.37 0.32...
-
A dam across a Maine river is going to be dismantled. Its original dimensions above the water on the downriver side of the dam are shown below. On the upriver side of the dam, the water level reaches...
-
Using the sampling distribution determined for = (X1 + X2)/2 in Exercise 7.5, verify that E[] = and sd() = /2.
-
Describe the content and purpose of a post-closing trial balance. LO8
-
How does treasury stock affect the authorized, issued, and outstanding shares? AppendixLO1
-
Consider the drug treatment system shown in the figure below. A hemispherical cluster of unhealthy cells is surrounded by a larger hemisphere of stagnant dead tissue (species B), which is turn...
-
Evan Company purchased a machine for $600,000 on August 1, 2010. Evan estimates the machine will have a ten-year useful life and a salvage value of $40,000. Evan calculates depreciation for a year to...
-
N 1 A New Kind of Structure Admit it. Sometimes the projects you're working on (school, work, or both) can get pretty boring and monotonous. Wouldn't it be great to have a magic button you could push...
-
If an oxidation occurs in a reaction, it must be accompanied by a reduction. Is the statement true? Explain why or why not.
-
Linking the energetically unfavorable reaction A B to a second, favorable reaction B C will shift the equilibrium constant for the first reaction. Is the statement true? Explain why or why not.
-
The following are the heights (in feet) and the number of stories of nine notable buildings in Houston. Use the data to construct a scatter plot. What type of pattern is shown in the scatter plot?...
-
Each of the accompanying graphs shows a do plot of data from three separate random samples for each of the four graphs, indicate whether you think that the basic assumptions for single-factor ANOVA...
-
Program Milestones Milestone #1 - Selection GUI - Create Account/Login/Cancel Obtain a copy of Eclipse and complete "Getting Started in Eclipse". Create your project in Eclipse and the package and...
-
= The momentum transfer is q = ph - Ph, where p is the hadron momentum after the collision. This relationship holds for the time as well as the space components, i.e., for the 4-vectors. Thus, we...
-
When should HR be the interviewer, and when should a hiring manager or co-workers be involved? Should reference checking be done before or after the interview? Would you ask during the interview any...
-
1. Monoclean Company manufactures a single product, Glamour. The standard cost specification sheet shows the following standards for one unit of Glamour: 8 kg of material M @ $6.5 per kg $52 4 hours...
-
Find a parametric representation for the surface. The part of the ellipsoid x 2 + 2y 2 + 3z 2 = 1 that lies to the left of the xz-plane.
-
Under what conditions is the following SQL statement valid?
-
Tabulate the natural log of the equilibrium constant as a function of temperature between 298 to 3000 K for the equilibrium reaction CO + H2O = CO2 + H2. Compare your results to those obtained by...
-
It is desired to control the amount of CO in the products of combustion of octane C8H18 so that the volume fraction of CO in the products is less than 0.1 percent. Determine the percent theoretical...
-
If the equilibrium constant for the reaction H2 + 1/2O2 H2O is K, the equilibrium constant for the reaction 2H2O 2H2 + O2 at the same temperature is (a) 1/K (b) 1/(2K) (c) 2K (d) K2 (e) 1/K2
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


