The following diagrams represent equilibrium mixtures for the reaction A 2 + B A + AB
Question:
The following diagrams represent equilibrium mixtures for the reaction A2 + B ⇌ A + AB at 300 K and 500K. The A atoms are red, and the B atoms are blue. Is the reaction exothermic or endothermic?
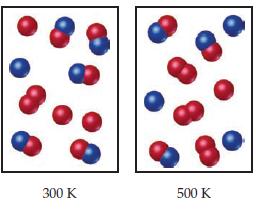
Transcribed Image Text:
300 K 500 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
In case of exothermic reaction number of produc...View the full answer

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
The following diagrams represent a hypothetical reaction A B, with A represented by red spheres and B represented by blue spheres. The sequence from left to right represents the system as time...
-
The following diagrams represent the equilibrium state for three different reactions of the type A + X AX (X = B, C, or D): (a) Which reaction has the largest equilibrium constant? (b) Which reaction...
-
The following diagrams represent mixtures of NO(g) and O2(g). These two substances react as follows: 2 NO(g) + O2(g) -- 2 NO2(g) It has been determined experimentally that the rate is second order in...
-
The chief accountant for Dickinson Corporation provides you with the following list of accounts receivable that were written off in the current year: Dickinson Corporation follows the policy of...
-
Steam enters an adiabatic nozzle steadily at 3 MPa, 670 K, 50 m/s, and exits at 2 MPa and 200 m/s. If the nozzle has an inlet area of 7 cm2. Determine The exit area.
-
How would the correlation between returns on a project and returns on the firm's other assets affect the project's risk? AppendixLO1
-
Write a model relating E(y) to one qualitative independent variable that is at four levels. Define all the terms in your model.
-
National Car Rental Systems, Inc., commissioned the U.S. Automobile Club (USAC) to conduct a survey of the general condition of the cars rented to the public by Hertz, Avis, National, and Budget...
-
help QUESTION MODERNE Ovom myckades 1 QUESTION Ambulance The bathrowing the period of the Other than the D. The concernance and they Gando 1 QUESTION 4 The correo Overcome in the proced Oweede Og...
-
What internal resources and assets did FreshDirect have that gave it a competitive advantage? How did FreshDirect compete? What competitive dynamics affect FreshDirect now? First launched in July...
-
When lead (IV) oxide is heated above 300C it decomposes according to the following reaction PbO 2 (s) PbO(s) + O 2 (g). Consider the two sealed vessels of PbO 2 shown here. If both vessels are...
-
Which of the following statements are true and which are false? (a) The equilibrium constant can never be a negative number. (b) In reactions that we draw with a single-headed arrow, the equilibrium...
-
Obtain the z-score that has area 0.70 to its right.
-
what methods ,to do ,or steps do you need to be sure to address when need to make a change in your organization that will help you navigate the organizational culture related to change? Especially...
-
How do you best receive information? Do you prefer written or oral reports? Shorter or longer briefings or reports? Quantitative or qualitative data? Formal or informal styles? How do you ensure...
-
Continuing with an examination of the laws in the state you've written about in earlier discussions, what state and local statutes exist that address the medical conditions or needs of eligible...
-
1. Think about the various soft drinks that you know from the local market and chose any 3 out of that ( e.g. Coca-Cola, Pepsi, 7-Up, Mirinda Citrus, Saudi Champagne, Shaani, Sun Top & Sun Cola,...
-
Leadership is an integral element in any job, regardless of the work title. However, it is important to recognize that leadership is not just one single skill; instead, success in leadership depends...
-
The piston of a machine exerts a constant force on a ball as it moves horizontally through a distance of 15 cm. You use a motion detector to measure the speed of five different balls as they come off...
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
Predict whether each of the following oxides is ionic or molecular: SnO2, Al2O3, CO2, Li2O, Fe2O3, H2O Explain the reasons for your choices.
-
Some metal oxides, such as Sc2O3, do not react with pure water, but they do react when the solution becomes either acidic or basic. Do you expect Sc2O3 to react when the solution becomes acidic or...
-
(a) What is meant by the terms acidic oxide and basic oxide? (b) How can we predict whether an oxide will be acidic or basic based on its composition?
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


