When lead (IV) oxide is heated above 300C it decomposes according to the following reaction PbO 2
Question:
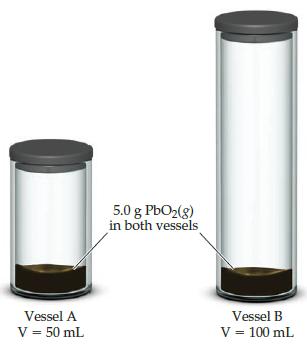
When lead (IV) oxide is heated above 300°C it decomposes according to the following reaction PbO2(s) ⇋ PbO(s) + O2(g). Consider the two sealed vessels of PbO2
shown here. If both vessels are heated to 400°C and allowed to come to equilibitum which of the following statements is true?
(a) There will be less PbO2 remaining in vessel A
(b) There will be less PbO2 remaining in vessel B
(c) The amount of PbO2 remaining in each vessel will be the same.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





