The three graphs below show the variation in radius, effective nuclear charge, and maximum oxidation state for
Question:
The three graphs below show the variation in radius, effective nuclear charge, and maximum oxidation state for the transition metals of period 4. In each part below identify which property is being plotted.
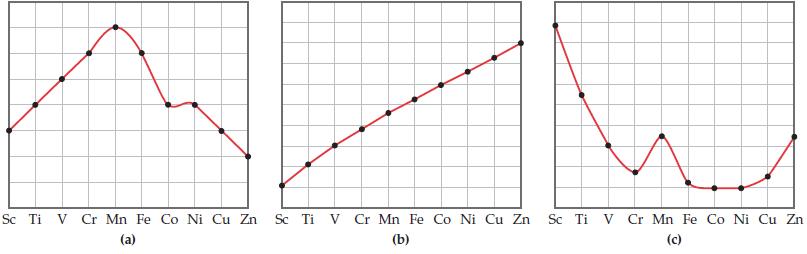
Sc Ti V Cr Mn Fe Co Ni Cu Zn Sc Ti V Cr Mn Fe Co Ni Cu Zn Sc Ti V Cr Mn Fe Co Ni Cu Zn (a) (b) (c)
Step by Step Answer:
a Maximum oxidation state dblock elements exhibit a variety of oxidation states A complete or halffu...View the full answer



Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Sciences questions
-
O n the same graph, plot the effective nuclear charge and atomic radius (see Figure 8.5) versus atomic number for the second period elements Li to Ne. Comment on the trends.
-
Consider the iso-electronic ions F- and Na+. (a) Which ion is smaller? (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening...
-
Consider the iso-electronic ions Cl- and K+. (a) Which ion is smaller? (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute nothing to the...
-
Consider thedeadlock situation that could occur in the dining-philosophers problem when the philosophers obtain the chopsticks one at a time. Discuss how the four necessary conditions for deadlock...
-
Would you expect heteroscedasticity to be present in the following regressions? Sample Net worth Log of net worth (a) Corporate profits Fortune 500 (b) Log of corporate (c) Dow Jones industria (o)...
-
Interpret calculator display: The following TI-84 Plus display presents the results of a hypothesis test. a. Is this a test for a mean, a proportion, or a standard deviation? b. What are the null and...
-
E18-3 Financial reporting during bankruptcyDistributions to creditors Noona Corporation files for Chapter 7 bankruptcy, when the book value of its net land and building is $80,000, and these assets...
-
Hammond Inc. experienced the following transactions for 2012, its first year of operations: 1. Issued common stock for $80,000 cash. 2. Purchased $225,000 of merchandise on account. 3. Sold...
-
Shamrock Inc. manufactures cycling equipment. Recently, the companys vice-president of operations has requested construction of a new plant to meet the increasing demand for the companys bikes. After...
-
Let U1, U2, . . . be a sequence of independent uniform (0, 1) random variables. In Example 5i we showed that, for 0 x 1,E[N(x)] = ex, where This problem gives another approach to establishing that...
-
Using Gf for ozone from Appendix C, calculate the equilibrium constant for Equation 22.24 at 298.0 K, assuming no electrical input. electricity 3 02(g) 2 03(g) AH 285 k %3D
-
The lanthanide contraction explains which of the following periodic trends? (a) The atomic radii of the transition metals first decrease and then increase when moving horizontally across each period....
-
When pseudoionone is treated with BF3 in acetic acid, ring closure takes place and α-and β-ionone are produced. This is the next step in the vitamin A synthesis. (a) Write...
-
3. Given the continuous beam shown below, which span or spans should be loaded with a uniform distributed load to produce a maximum moment at support B? (5 points) SPAN 1 SPAN 2 SPAN 3 A B D 20 ft...
-
Complete the following writing assignment: Analyze the attached 10_pages. Write_about them, summarize what you read, and connect it to personal experiences. CHAPTER 8 Anxiety Disorders DAVID P....
-
As a manager of an airline company you want to learn the average weight of luggages checked in on a flight. From a sample of 1 6 luggages, you find the average to be 2 6 kg and the standard deviation...
-
What is the Manufacturing Cycle Efficiency? 11. Use High-Low to find the fixed and variable costs. Machine Month Costs Hours 12345678 $1,730,890 15,820 $1,753,860 13,980 $1,562,890 11,550 4...
-
Jimmy Padilla purchased a gravel pit in the current year for $944,232 and estimates that there will be a residual value in the land of $36,404 once resource extraction is complete. He estimates that...
-
Laurie bought a home in 2017 for $65,000. On November 2, 2020, she sells it for $114,000. Laurie uses the proceeds to purchase a duplex costing $200,000. She uses one unit in the duplex as her...
-
Find the intercepts and then graph the line. (a) 2x - 3y = 6 (b) 10 - 5x = 2y
-
Referring to Figure 11.28, describe all the phase changes that would occur in each of the following cases: (a) Water vapor originally at 0.005 atm and - 0.5 oC is slowly compressed at constant...
-
The molecules Have the same molecular formula (C3H8O) but different normal boiling points, as shown. Rationalize the difference in boiling points? (b) Ethyl methyl ether (a) Propanol 97.2C 10.8 C
-
Referring to Figure 11.29, describe the phase changes (and the temperatures at which they occur) when CO2 is heated from - 80 oC to - 20 oC at (a) A constant pressure of 3 atm, (b) A constant...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


