This figure shows the interaction of a cation with surrounding water molecules. Would you expect the energy
Question:
This figure shows the interaction of a cation with surrounding water molecules.
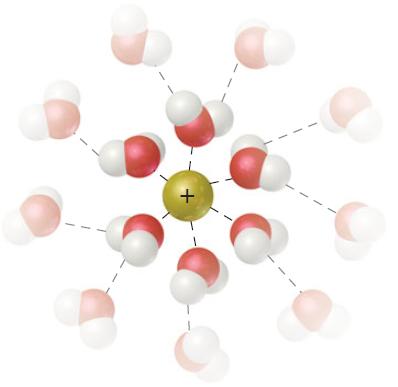
Would you expect the energy of ion–solvent interaction to be greater for Na+ or Li+? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
Ionsolvent interactions inversely proportion al to the size of the ion Ie smaller is ...View the full answer

Answered By

Umair Expert
Hi Everyone.
I have 6 years of teaching experience.
I am serving as a tutor 2 more websites.
I will provide you projects and solutions of questions related to any subject.
I am a good programmer.(PHP, Python)
I am good command in mathematics.
I am here to assist you.
I'll be very happy to work with you...
Thanks dear..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:
Students also viewed these Sciences questions
-
Which of the following molecules would you expect to be aromatic? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) N+
-
Would you expect alanine (an amino acid) to be more soluble in water or in hexane? Explain.
-
The accompanying graph depicts the interaction energy between two water molecules situated so that their dipole moments are parallel and pointing in the same direction. Sketch an approximate curve...
-
The Federal Reserve may raise its benchmark interest rate later this month. How is this achieved? Why would they do this? Explain the consequences fully. (Include graphs with your answer)
-
Find and s for: (a) The lizard data in Exercise 2.19. (b) The acid rain data in Exercise 2.115. 1.28 1.36 1.24 2.47 1.94 2.52 2.67 1.29 1.56 2.66 2.17 57 2.10 2.54 163 2.11 2.57 1.72 0.76 1.02 1.78...
-
You are the project manager for your organization and are reviewing the requirements for your new project. The business analyst has completed the requirements and is consulting with you on what each...
-
What are the design strategies that Galbraith describes? LO.1
-
Use the following information for a manufacturer to compute cost of goods manufactured and cost of goodssold: Beginning Ending Inventories: Raw Materials Work-in-Process Finished Goods Other...
-
SCENARIO A Answer the questions in the following scenario. Be sure to show all your working. The scenario is for the 2017/18 financial year. You will need to refer to the Australian Taxation Offices...
-
Food scientists have created a new oil. At room temperature, the oil is a liquid. As the oil gets colder however, it stiffens (thickens) into a sticky gel. To explore the properties of the oil, the...
-
What molecular structural features cause high-density polyethylene to be denser than low-density polyethylene?
-
List four properties of a solution that depend on the total concentration but not the type of particle or particles present as solute.Write the mathematical expression that describes how each of...
-
How do changes such as demand changes, new pollution control laws, the changing value of currency, and price changes affect operations? Name specific impacts on operations and the supply chain for...
-
Archer Contracting repaved 50 miles of two-lane county roadway with a crew of six employees. This crew worked 8 days and used \($7,000\) worth of paving material. Nearby, Bronson Construction repaved...
-
An insurance company has the following profitability analysis of its services: The fixed costs are distributed equally among the services and are not avoidable if one of the services is dropped. What...
-
The Scantron Company makes bar-code scanners for major supermarkets. The sales staff estimates that the company will sell 500 units next year for 10,000 each. The production manager estimates that...
-
Determine the following: a. The stockholders equity of a company that has assets of \(\$ 625,000\) and liabilities of \(\$ 310,000\). b. The retained earnings of a company that has assets of \(\$...
-
You are the manager of internal audit for Do-It-All, Ltd., a large, diversified, decentralized manufacturing company. Over the past two years, the information systems function in Do-It-All has...
-
Prove each equation. (a) Equation 19a (b) Equation 19b (c) Equation 19c 19a sin x cos y = [sin(x + y) + sin(x - y)] 19b cos x cos y =[cos(x + y) + cos(x - y)] 19c sin x sin y = [cos(x - y) - cos(x +...
-
Organizations are increasing their use of personality tests to screen job applicants. What are some of the advantages and disadvantages of this approach? What can managers do to avoid some of the...
-
Air in an automobile tire is maintained at a pressure of 220 kPa (gauge) in an environment where the atmospheric pressure is 94 kPa. The air in the tire is at the ambient temperature of 25C. Now a...
-
The thrust developed by the engine of a Boeing 777 is about 380 kN. Assuming choked flow in the nozzle determine the mass flow rate of air through the nozzle. Take the ambient conditions to be 265 K...
-
A stationary temperature probe inserted into a duct where air is flowing at 250 m/s reads 85C. What is the actual temperature of air?
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


