Question: In 1911, Ernest Rutherford discovered the basic structure of the atom by shooting positively charged alpha particles with a speed of 107 m per sec
In 1911, Ernest Rutherford discovered the basic structure of the atom by "shooting" positively charged alpha particles with a speed of 107 m per sec at a piece of gold foil 6 × 10-7 m thick. Only a small percentage of the alpha particles struck a gold nucleus head-on and were deflected directly back toward their source. The rest of the particles often followed a hyperbolic trajectory because they were repelled by positively charged gold nuclei. As a result of this famous experiment, Rutherford proposed that the atom was composed mostly of empty space with a small and dense nucleus.
The figure shows an alpha particle A initially approaching a gold nucleus N and being deflected at an angle u = 90°. N is located at a focus of the hyperbola, and the trajectory of A passes through a vertex of the hyperbola.

(a) Determine the equation of the trajectory of the alpha particle if d = 5 × 10-14 m.
(b) What was the minimum distance between the centers of the alpha particle and the gold nucleus? Write the answer using scientific notation. Round to the nearest tenth.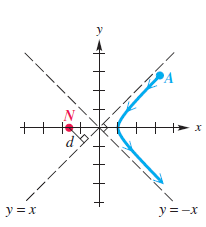
Ernest Rutherford P. y =x y=-x - --
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
a We must determine a and b in the equation1 The asymptotes are yx and y x which have slopes of 1 an... View full answer

Get step-by-step solutions from verified subject matter experts


