Apply the loop rule to loop aedcba in Figure 21.25. R 2.5 a 12 12 13
Question:
Apply the loop rule to loop aedcba in Figure 21.25.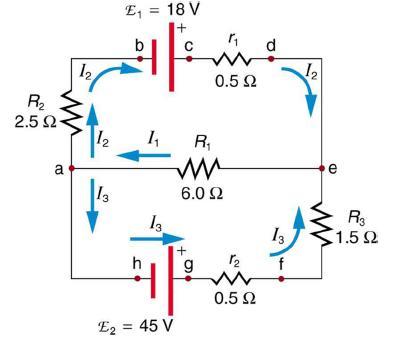
Transcribed Image Text:
R₂ 2.5 Ω· a 12 12 13 E₁ = 18 V C F b h 1₁ 13 R₁ ww 6.0 92 E2 = 45 V 0.5 Ω + 12 0.5 Ω d 13 f 1₂ e R3 1.592
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Kirchhoffs loop rule It states that the sum of all the electri...View the full answer

Answered By

Anurag Agrawal
I am a highly enthusiastic person who likes to explain concepts in simplified language. Be it in my job role as a manager of 4 people or when I used to take classes for specially able kids at our university. I did this continuously for 3 years and my god, that was so fulfilling. Sometimes I've skipped my own classes just to teach these kids and help them get their fair share of opportunities, which they would have missed out on. This was the key driver for me during that time. But since I've joined my job I wasn't able to make time for my passion of teaching due to hectic schedules. But now I've made a commitment to teach for at least an hour a day.
I am highly proficient in school level math and science and reasonably good for college level. In addition to this I am especially interested in courses related to finance and economics. In quest to learn I recently gave the CFA level 1 in Dec 19, hopefully I'll clear it. Finger's crossed :)
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Apply the loop rule to loop afedcba in Figure 21.45. a R ww 200 0.50 18 V P www 15 2 P 0 1 6 2 g 3.0V 0.25 02 R w 82 24VI 12V 0.5 (2 " m d 0.75 22
-
Apply the loop rule to loop akledcba in Figure 21.50. R 5.0 a R 78 b 14 T+ = 24.0 V 0.10 = 48.00 k 12 0.50 g = 60V Pa M 1.0.05 h R 40 R 20 FA 70.20 2 = 36.0 V g
-
Apply the loop rule to loop abcdefgha in Figure 21.25. R 2.5 a 12 12 E = 18 V b C 4F h 1 13 R ww 6.0 92 E2 = 45 V 0.5 + 12 0.5 d 13 f 12 e R3 1.592
-
The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH20H------->cat CH3CH=CHCH3 + H2O The rate law is -r Bu = KPBU/(1+KBuPBul with k= 0.054...
-
Briefly describe the structural changes that accompanied the renaming of the IASC to the IASB.
-
Consider the following simple monetary policy rule: In the following questions, you are asked to gather data on inflation and short-run output to feed into this policy rule. A good resource for the...
-
When does a dividend become a companys legal obligation? AppendixLO1
-
Wallace Corporation summarizes the following information from its weekly payroll records during April. Prepare the two journal entries to record the payment of the payroll and the accrual of its...
-
I NEED HELP TO SOLVE ONE PROBLEM Use the adjusted trail balance to prepare an income statement, a statement of changes in equity and a balance sheet. Assume that the owner, Jeff Moore, made no owner...
-
Apply the loop rule to loop abcdefghija in Figure 21.50. R 5.0 a R 78 b 14 Ta , = 24.0 V im 0.10 = 48.00 k 12 0.50 g = 60 V M 10.05 h | R 40 R - 20 TA 70.20 g 2. = 36.0 V
-
What is the approximate sound intensity level in decibels of a 600-Hz tone if it has a loudness of 20 phons? If it has a loudness of 70 phons?
-
A United Kingdombased financial analyst considers a Z-score model in evaluating two publicly traded non-manufacturing companies as follows: Z-Score Model = 1.2 A + 1.4 B + 3.3 C + 0.6 D + 0.999 ...
-
Ginger Tyler comes into Johns Medical Center for her routine office visit. Her co-payment is $50.00. She hands the office manager $60.00. The $10.00 change should be taken from which cash management?...
-
Do you believe that the labour laws that are currently in place (i.e., the Ontario Labour Relations Act) are sufficient to guarantee workers have adequate voice and equity in the workplace? Explain...
-
The DSV Partnership decided to liquidate as of June 30, 20X5. Its balance sheet as of this date follows: Assets Cash Accounts Receivable (net) Inventories DSV PARTNERSHIP Balance Sheet At June 30,...
-
Below what IQ does .27 of the population fall if the mean is 100 with a standard deviation of 15? (Don't round off IQ score.)
-
1. Can modern day roles be placed in the paradigm of masters, overseers, drivers, and slaves? If so, describe a parallel to these relationships you could interpret through this type of lens. If not,...
-
What processes would you recommend for die sinking in a die block, such as that used for forging? Explain.
-
The Taylor's series expansion for cosx about x = 0 is given by: where x is in radians. Write a user-defined function that determines cosx using Taylor's series expansion. For function name and...
-
A 0.030-kg lead bullet hits a steel plate, both initially at 20oC. The bullet melts and splatters on impact. (This action has been photographed.) Assuming that of the bullets kinetic energy goes into...
-
The temperature of a lead block and a copper block, both 1.0 kg and at 20 oC, is to be raised to 100 oC. (a) The copper will require (1) more heat, (2) the same heat, (3) less heat than the lead....
-
A cyclist with a total skin area of 1.5 m2 is riding a bicycle on a day when the air temperature is 20oC and her skin temperature is 34oC. The cyclist does work at about 200 W (moving the pedals) but...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


