Verify that the total number of nucleons, total charge, and electron family number are conserved for each
Question:
Verify that the total number of nucleons, total charge, and electron family number are conserved for each of the fusion reactions in the proton proton cycle in
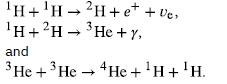
Transcribed Image Text:
H+H 1H+2H 2H+e+ + Ve He+y, and 3 He+ 3 He He+H+H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Conservation Laws in ProtonProton Cycle Reactions Youre right Lets verify that the total number of n...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
(a) Calculate the energy released in the neutron-induced fission (similar to the spontaneous fission in Example 32.3) (b) This result is about 6 MeV greater than the result for spontaneous fission....
-
(a) Calculate the energy released in the neutron-induced fission reaction n +239 Pu 96 Sr + 140Ba + 4n, given m(96Sr) = 95.921750 u and m(140Ba) = 139.910581 u. (b) Confirm that the total number of...
-
Confirm that charge, electron family number, and the total number of nucleons are all conserved by the rule for electron capture given in the equation To do this, identify the values of each before...
-
Calculate the binding energy per nucleon for a 14/7N nucleus.
-
"That old equipment for producing subassemblies is worn out," said Paul Taylor, president of Timkin Company. "We need to make a decision quickly" The company is trying to decide whether it should...
-
A very conscientious and quality driven tire retailer (Todd Witt Tires) is in the process of determining which tire supplier to use to purchase tires, which will be sold to end customers. Todd Witt...
-
6. If growth is a significant value driver, does getting bigger translate into creating value? Explain.
-
Yoto Heavy Industrial uses ten units of Part No. T305 each month in the production of large diesel engines. The cost to manufacture one unit of T305 is presented below: Direct material...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
Berkley Corp. wanted to buy 1,000 customized umbrellas imprinted with their logo to use for promotional purposes. It planned to use 250 of the umbrellas for an event scheduled for early February 2012...
-
To improve the reliability of the channel described in the last example, we repeat each digit in the message three times. What is the probability that 111 was sent given that (a) we received 101?...
-
What is the fundamental difference in the price-level adjustments made under current cost accounting and under historical cost/constant purchasing power accounting?
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Use values of r cov (Table 17.1) to estimate the XY bond lengths of ClF, BrF, BrCl, ICl and IBr. Compare the answers with values in Fig. 17.8 and Table 17.3, and comment on the validity of the method...
-
From a square whose side has length \(x\), measured in meters, create a new square whose side is \(10 \mathrm{~m}\) longer. Find an expression for the sum of the areas of the two squares as a...
-
From a square whose side has length \(x\), measured in inches, create a new square whose side is 5 in. longer. Find an expression for the difference between the areas of the two squares as a function...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A circle with radius 5
-
What is the effect on EOQ and total cost of the following types of changes for the data in problem 1? a. A 40 percent increase in demand. b. A 20 percent increase in carrying charge. c. Use a...
-
A local politician is concerned that a program for the homeless in her city is discriminating against blacks and other minorities. The following data were taken from a random sample of black and...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


