A glass tube contains 2 x 10 11 atoms, some of which are in the ground state
Question:
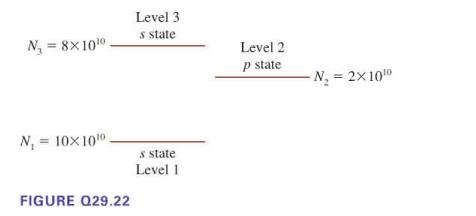
A glass tube contains 2 x 1011 atoms, some of which are in the ground state and some of which are excited. Figure Q29.22 shows the populations for the atoms' three energy levels. Is it possible for these atoms to be a laser? If so, on which transition would laser action occur? If not, why not?
Transcribed Image Text:
= 8×10¹0. N₁ N₁ = 10x10¹0. FIGURE Q29.22 Level 3 s state s state Level 1 Level 2 p state -N₂ = 2x10¹0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
No it is not possible for these atoms to be a laser For a laser to exis...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321595492
2nd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:
Students also viewed these Physics questions
-
The cosmic dawn that preceded the epoch of reionization can be probed by low frequency CMB observations using a special radio hyperfine line emitted and absorbed by hydrogen atoms. This line is...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Discuss the primary sources of nonverbal communication.
-
Enid Co., a womens clothing store, purchased $7,500 of merchandise from a supplier on account, terms FOB destination, 2/10, n/30. Enid Co. returned $1,200 of the merchandise, receiving a credit...
-
Using substantial factor analysis, who are the possible parties to be charged with causation, and why?
-
LO2 Explain how the related party construct and the arms-length transaction concept interact.
-
The number of megapixels in a digital camera is one of the most important factors in determining picture quality. But do digital cameras with more megapixels cost more? The following data show the...
-
Which of the following funds can be used to account for the spendable income from a Private-Purpose Trust Fund? Select one: O O O a. Custodial Fund b. General Fund c. Capital Projects Fund d. Pension...
-
An atom emits a photon with a wavelength of 275 nm. By how much does the atom's energy change? A. 0.72 eV B. 1.06 eV C. 2.29 eV D. 3.06 eV E. 4.51 eV
-
The angular momentum of an electron in a Bohr hydrogen atom is 3.18 10 -34 kg. m 2 /s. What is the atom's energy? A. - 13.60 eV B. -6.73 eV C. -3.40 eV D. -1.51 eV E. -0.47 eV
-
There are many open-source as well as freeware applications that shouldnt be overlooked. Compare Microsoft Word with OpenOffice and Google Docs. What are the main differences between the...
-
How do we design a superconducting induction motor?
-
How do we design a superconducting cyclo converter?
-
How do we design a superconducting non-inverting op amp circuit?
-
What sutra is used to verify BODMAS principle in Vedic Mathematics?Explain in brief.
-
What Is Accounting? Definition, Types, History, & Examples
-
If you are given an empty bucket like the one in Fig. 1.5.8, having a hole in the bottom from which all the water has leaked, can you tell how long ago the bucket was full? Of course not, and the...
-
The sales department of P. Gillen Manufacturing Company has forecast sales in March to be 20,000 units. Additional information follows: Finished goods inventory, March 1 . . . . . . . . . . . . . . ....
-
Figure Q16.4 shows a snapshot graph and a history graph for a wave pulse on a stretched string. They describe the same wave from two perspectives. a. In which direction is the wave traveling?...
-
Draw the history graph D(x = 4.0 m, t) at x = 4.0 m for the wave shown in FIGURE EX16.4. D (cm) 14 -x (m) 5 6 7 1 2 3 -1| 1.0 m/s Snapshot graph of a wave at f = 2 s FIGURE EX16.4
-
Rank in order, from largest to smallest, the wavelengths a , b , and c for sound waves having frequencies f a = 100 Hz, f b = 1000 Hz, and f c = 10,000 Hz. Explain.
-
Wildhorse Inc. issued $6 million of 10-year, 8% convertible bonds on June 1, 2020, at 99 plus accrued interest. The bonds were dated April 1, 2020, with interest payable April 1 and October 1. Bond...
-
The following details are provided by a manufacturing company. Investment Useful life Estimated annual net cash inflows for first year Estimated annual net cash inflows for second year Estimated...
-
Waterways Corporation is preparing its budget for the coming year, 2020. The first step is to plan for the first quarter of that coming year. The company has gathered information from its managers in...

Study smarter with the SolutionInn App


