A glass capillary tube with a radius of 0.050 mm is inserted into an open container filled
Question:
A glass capillary tube with a radius of 0.050 mm is inserted into an open container filled with an unknown liquid, and the meniscus has an appearance similar to Figure 10.35A. It is found that the surface tension causes the liquid in the tube to rise a distance of 2.5 cm relative to the surface of the liquid outside the tube. If the density of the liquid is 1000 kg/m1what is the surface tension?
Figure 10.35A
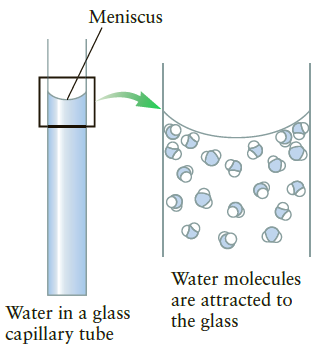
Meniscus Water molecules are attracted to the glass Water in a glass capillary tube
Step by Step Answer:
The pressure on the liquid inside is the capillary pressur...View the full answer



College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano
Related Video
The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface of a glass of water do not have other water molecules on all sides of them and consequently, they cohere more strongly to those directly associated with them (in this case, next to and below them, but not above). is not really true that a \\\"skin\\\" forms on the water surface; the stronger cohesion between the water molecules as opposed to the attraction of the water molecules to the air makes it more difficult to move an object through the surface than to move it when it is completely submersed
Students also viewed these Sciences questions
-
A glass capillary tube with a diameter of 0.50 mm and length 10 cm is filled with a salt solution with a resistivity of 0.10m. What is the resistance?
-
Figure P6.6 shows an angled, glass capillary tube with diameter 110 m. Other dimensions are shown. (a) Where will the top of the capillary rise be? (b) What is the water pressure in the horizontal...
-
The contact angle for water on clean glass is close to zero. Calculate the surface tension of water at 30C given that at that temperature water climbs to a height of 9.11 cm in a clean glass...
-
Alleghany Community College operates four departments. The square footage used by each department is shown below. Alleghany's annual building rental cost is $320,000 What amount of rent expense that...
-
Prepare an amortization schedule for a three-year loan of $75,000. The interest rate is 8 percent per year, and the loan calls for equal annual payments. How much interest is paid in the third year?...
-
Two different routes are under consideration for a new interstate highway: TABLE P16.13 For either route, the volume of traffic will be 400,000 cars per year. These cars are assumed to operate at...
-
What is the decision facing Alibaba? When youre already big it can be hard to get bigger. Thats why Alibaba is working hard to continue to expand its e-tailing empire. In 1999, Jack Ma, inspired by a...
-
Optimum Health Inc. provides diet, fitness, and nutrition services to clients who want a healthier lifestyle. The company customizes a program for each client based on their individual goals that...
-
What segments would Link need to report for 2023 ? How would they be reported
-
At the beginning of 2013, P & D Enterprises had the following balances in its accounts: Cash .......... $16,800 Inventory ........ 4,000 Land .......... 2,000 Common stock ...... 12,000 Retained...
-
Repeat Problem 72, but now for a tube of radius 10 nm. Express your answer in pascals and as a ratio relative to atmospheric pressure. Data from Problem 72 What is the capillary pressure for water in...
-
The viscosity of normal (healthy) blood is about three times greater than the viscosity of water. Certain diseases such as polycythemia can cause the viscosity of blood to be as much as three times...
-
Using the standard normal table, determine a z value (to two decimal places) such that the a. Cumulative area to z is 0.7486. b. Cumulative area to z is 0.0735. c. Area between z and positive...
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Given the following HTML, write a simple bit of JavaScript code that will DELETE ALL OF THE TAGS ON THE PAGE. Quiz I'm a Heading I'm a paragraph I'm special I'm also a paragraph Footer! HINT: You'll...
-
Your company has been quite successful in sending employees on international assignments. As the HR Manager responsible for selecting such employees, present a report to the management of your...
-
You will be looking at a particular market in the economy. I will assign the market to you arbitrarily. Please look for at the end of this document to identify which market you will be responsible...
-
Fosters Manufacturing Co. warrants its products for one year. The estimated product warranty is 2% of sales. Assume that sales were $1,800,000 for January. On February 7, a customer received warranty...
-
a. Show that the expansion of q(x) in ascending powers of x can be approximated to 10 2x + Bx 2 + Cx 3 where B and C are constants to be found. b. Find the percentage error made in using the series...
-
Are the effective nuclear charges listed in Figure 21.13 helpful in explaining the trend in the electron affinity with increasing atomic number? Explain your answer. 1s 1 1s 1.69 Li 2s 1.28 2s 1.91...
-
Are the effective nuclear charges listed in Figure 21.13 helpful in explaining the trend in the first ionization energy with increasing atomic number? Explain your answer. 1s 1 1s 1.69 Li 2s 1.28...
-
The electron affinities of He, Be, and Ne are negative, meaning that the negative ion is less stable than the neutral atom. Explain why this is so for these three elements.
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...

Study smarter with the SolutionInn App


