Question: A quantum system (a molecule) has the energy levels shown in Figure P29.59. A large number of these molecules in their ground states make up
A quantum system (a molecule) has the energy levels shown in Figure P29.59. A large number of these molecules in their ground states make up a gas.
(a) Suppose we want to excite these molecules to the state labeled E2 by shining light on the gas. What wavelength of light should we use?
(b) If we expose the molecules to a continuous spectrum of light, photons of particular wavelengths might be absorbed. What wavelength photons would you expect to be absorbed? Do not assume the molecules all start in their ground state. Explain why that assumption makes sense.
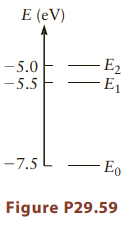
Step by Step Solution
There are 3 Steps involved in it
The photons absorbed in each case must have the energies that give them the energy difference b... View full answer

Get step-by-step solutions from verified subject matter experts


