Suppose a certain ionized atom produces an emission line in accordance with the Bohr model, but the
Question:
Suppose a certain ionized atom produces an emission line in accordance with the Bohr model, but the number of protons in the nucleus Z is not known. A group of lines in the spectrum forms a series in which the shortest wavelength is 22.8 nm and the longest is 41.0 nm. Atoms with Z greater than 1 can be described approximately by a modified version of Equations 29.15 and 29.16, which can be written as
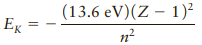
Find the second longest wavelength in this series of lines.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano
Question Posted:





