Question: In the spectrometer shown in Figure P24.67, do the ions have positive or negative charge? A. Positive B. Negative. If you have a sample of
In the spectrometer shown in Figure P24.67, do the ions have positive or negative charge?
A. Positive
B. Negative.
If you have a sample of unknown composition, a first step at analysis might be a determination of the masses of the atoms and molecules in the sample. A mass spectrometer to make such an analysis can take various forms, but for many years the best technique was to determine the masses of ionized atoms and molecules in a sample by observing their circular paths in a uniform magnetic field, as illustrated in Figure P24.67. A sample to be analyzed is vaporized, then singly ionized. The ions are accelerated through an electric field, and ions of a known speed selected. These ions travel into a region of uniform magnetic field, where they follow circular paths. An exit slit allows ions that have followed a particular path to be counted by a detector, producing a record of the masses of the particles in the sample.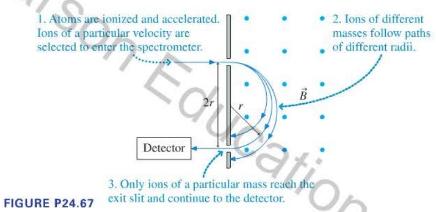
1. Atoms are ionized and accelerated. Ions of a particular velocity are selected to enter the spectrometer. 2. Ions of different masses follow paths of different radii. Edation Detector 3. Only ions of a particular mass reach the FIGURE P24.67 exit slit and continue to the detector.
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


