The spin s of an electron is a type of angular momentum. It is quantized and is
Question:
The spin s of an electron is a type of angular momentum. It is quantized and is equal to s = ±1/2ℏ, where the symbol h with a slash through the top is the reduced Planck’s constant ℏ = h/(2π), a quantity often found in equations concerning spin. As discussed in Section 28.4, a positive value of s denotes “spin up” orientation and a negative value means “spin down.” This intrinsic property determines the behavior of an electron under the influence of the magnetic field in a Stern–Gerlach experiment. Spin is related to the magnetic moment, which is given by the expression
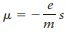
where e and m are the charge and mass of the electron, respectively.
(a) Find the value of the magnetic moment of the electron. It is a fundamental quantity called the Bohr magneton.
(b) Show that the units of the Bohr magneton can be expressed as J/T (joules per tesla).
Step by Step Answer:

College Physics Reasoning and Relationships
ISBN: 978-0840058195
2nd edition
Authors: Nicholas Giordano





