Question: Explain how the principles of quantum mechanics are able address the issues. The development of quantum mechanical theory stems from several issues that could not
Explain how the principles of quantum mechanics are able address the issues.
The development of quantum mechanical theory stems from several issues that could not be addressed by the principles of classical mechanics. From your reading of the assigned text as well as other literatures available on the topic, present one phenomenon of the physical world as we know today which is in conflict or can not be explained by classical mechanics alone. Explain how the principles of quantum mechanics are able address the issues
Use your 0wn words to show your understanding (If you are picking a phenomenon based on a fictional movie, attach a short clip and make sure that the basis are the real scientific concepts, not fictional concepts).
My notes



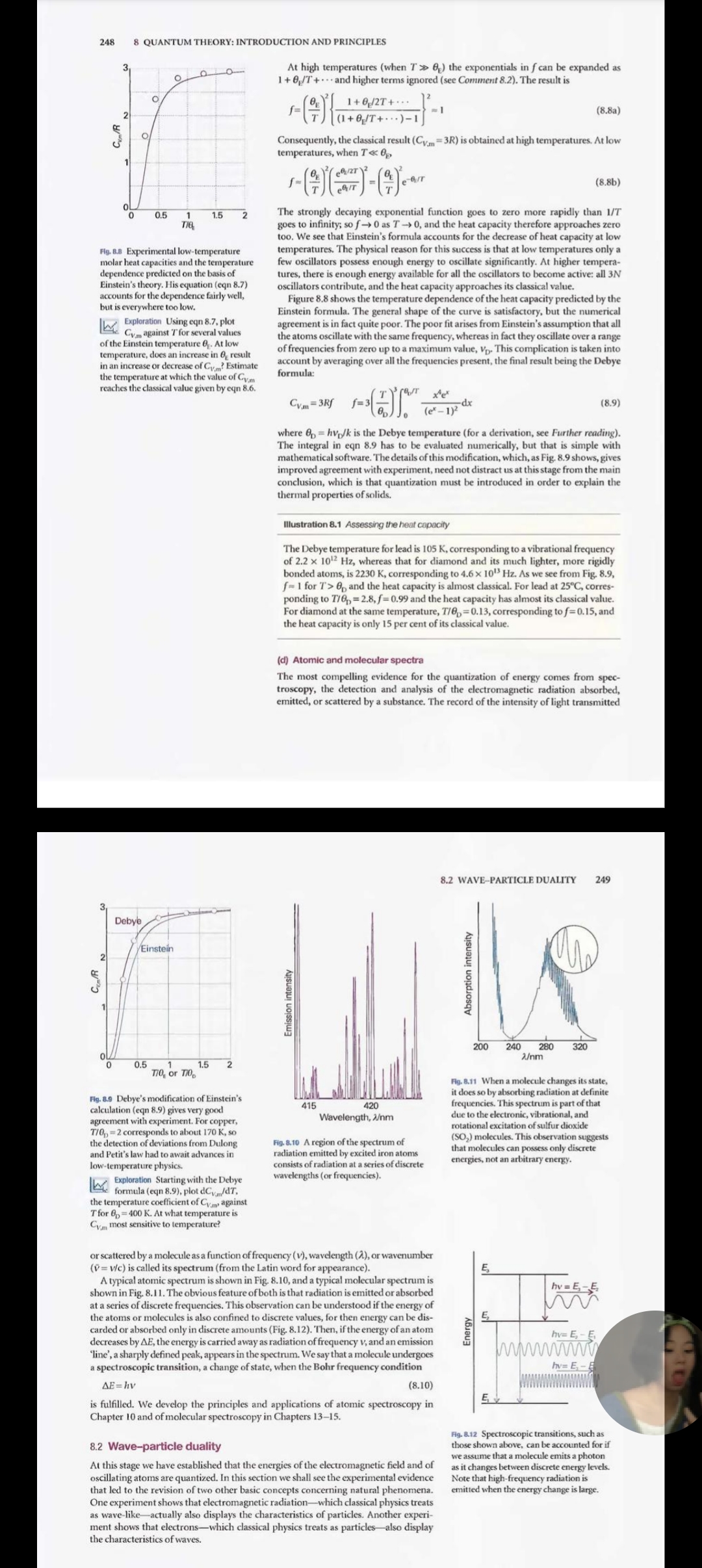
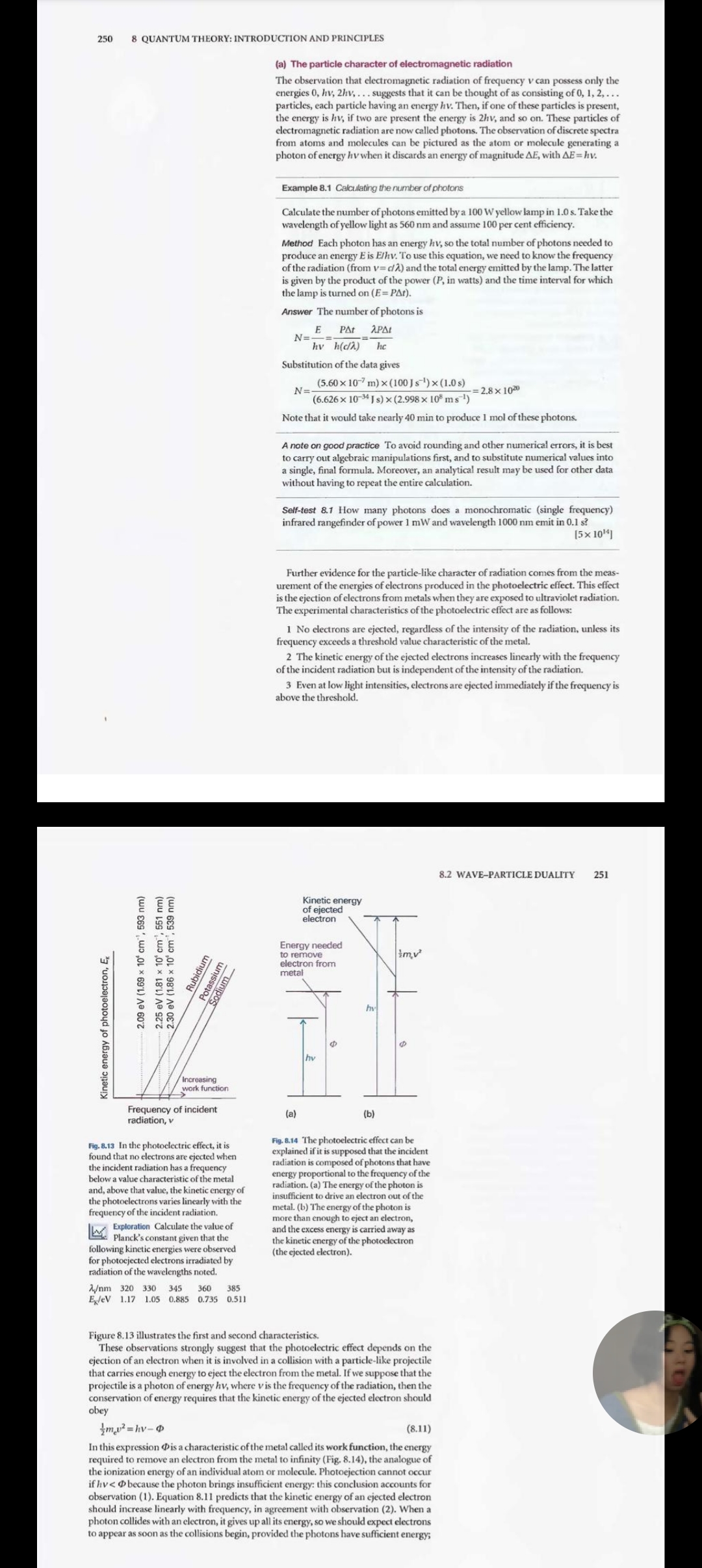



Quantum theory: introduction and principles This chapter introduces some of the basic principles of quantum mechanics. First, it reviews The origins of quantum the experimental results that overthrew the concepts of classical physics. These experi- mechanics ments led to the conclusion that particles may not have an arbitrary energy and that the classical concepts of 'particle' and 'wave' blend together. The overthrow of classical 8.1 The failures of classical physics mechanics inspired the formulation of a new set of concepts and led to the formulation of 8.2 Wave-particle duality quantum mechanics. In quantum mechanics, all the properties of a system are expressed 18.1 Impact on biology: Electron in terms of a wavefunction that is obtained by solving the Schrodinger equation. We see microscopy how to interpret wavefunctions. Finally, we introduce some of the techniques of quantum mechanics in terms of operators, and see that they lead to the uncertainty principle, one of The dynamics of microscopic the most profound departures from classical mechanics. systems 8.3 The Schrodinger equation It was once thought that the motion of atoms and subatomic particles could be 8.4 The Born interpretation of the expressed using classical mechanics, the laws of motion introduced in the seven- wavefunction teenth century by Isaac Newton, for these laws were very successful at explaining the motion of everyday objects and planets. However, towards the end of the nineteenth Quantum mechanical principles century, experimental evidence accumulated showing that classical mechanics failed when it was applied to particles as small as electrons, and it took until the 1920s to 8.5 The information in a discover the appropriate concepts and equations for describing them. We describe the wavefunction concepts of this new mechanics, which is called quantum mechanics, in this chapter, 8.6 The uncertainty principle and apply them throughout the remainder of the text. 8.7 The postulates of quantum mechanics The origins of quantum mechanics Checklist of key ideas Further reading The basic principles of classical mechanics are reviewed in Appendix 2. In brief, they Discussion questions show that classical physics (1) predicts a precise trajectory for particles, with precisely specified locations and momenta at each instant, and (2) allows the translational, Exercises rotational, and vibrational modes of motion to be excited to any energy simply by Problems controlling the forces that are applied. These conclusions agree with everyday experi- ence. Everyday experience, however, does not extend to individual atoms, and careful experiments of the type described below have shown that classical mechanics fails when applied to the transfers of very small energies and to objects of very small mass. We shall also investigate the properties of light. In classical physics, light is described as electromagnetic radiation, which is understood in terms of the electromagnetic field, an oscillating electric and magnetic disturbance that spreads as a harmonic wave through empty space, the vacuum. Such waves are generated by the acceleration of electric charge, as in the oscillating motion of electrons in the antenna of a radio transmitter. The wave travels at a constant speed called the speed of light, c, which 244 8 QUANTUM THEORY: INTRODUCTION AND PRINCIPLES Wavelength, 2 is about 3 x 10 m s". As its name suggests, an electromagnetic field has two com- ponents, an electric field that acts on charged particles (whether stationary or moving) and a magnetic field that acts only on moving charged particles. The elec- tromagnetic field is characterized by a wavelength, 2 (lambda), the distance between ( a) the neighbouring peaks of the wave, and its frequency, v (nu), the number of times per second at which its displacement at a fixed point returns to its original value (Fig. 8.1). The frequency is measured in hertz, where 1 Hz= 1 s". The wavelength and frequency of an electromagnetic wave are related by av=c (8.1) (b) Therefore, the shorter the wavelength, the higher the frequency. The characteristics of the wave are also reported by giving the wavenumber, V (nu tilde), of the Fig. 8.1 The wavelength, 2, of a wave is the radiation, where peak-to-peak distance. (b) The wa244 8 QUANTUM THEORY: INTRODUCTION AND PRINCIPLES Wavelength, 2 is about 3 x 105 m s". As its name suggests, an electromagnetic field has two com- ponents, an electric field that acts on charged particles (whether stationary or moving) and a magnetic field that acts only on moving charged particles. The elec- tromagnetic field is characterized by a wavelength, 2 (lambda), the distance between the neighbouring peaks of the wave, and its frequency, v (nu), the number of times per second at which its displacement at a fixed point returns to its original value (Fig. 8.1). The frequency is measured in hertz, where 1 Hz= 1 s". The wavelength and frequency of an electromagnetic wave are related by Av=c (8.1) Therefore, the shorter the wavelength, the higher the frequency. The characteristics of the wave are also reported by giving the wavenumber, V (nu tilde), of the Fig. 8.1 The wavelength, 2, of a wave is the radiation, where peak-to-peak distance. (b) The wave is shown travelling to the right at a speed c D= [8.2] At a given location, the instantaneous 2. amplitude of the wave changes through a complete cycle (the four dots show half a Wavenumbers are normally reported in reciprocal centimetres (cm '). cycle). The frequency, V, is the number of Figure 8.2 summarizes the electromagnetic spectrum, the description and classi- cycles per second that occur at a given fication of the electromagnetic field according to its frequency and wavelength. White point. light is a mixture of electromagnetic radiation with wavelengths ranging from about 380 nm to about 700 nm (1 nm = 10 m). Our eyes perceive different wavelengths of radiation in this range as different colours, so it can be said that white light is a Comment 8.1 mixture of light of all different colours. Harmonic waves are waves with The wave model falls short of describing all the properties of radiation. So, just as displacements that can be expressed as our view of particles (and in particular small particles) needs to be adjusted, a new sine or cosine functions. The physics of waves is reviewed in Appendix 3. view of light also has to be developed. 8.1 The failures of classical physics In this section we review some of the experimental evidence that showed that several concepts of classical mechanics are untenable. In particular, we shall see that observa- tions of the radiation emitted by hot bodies, heat capacities, and the spectra of atoms and molecules indicate that systems can take up energy only in discrete amounts (a) Black-body radiation A hot object emits electromagnetic radiation. At high temperatures, an appreciable proportion of the radiation is in the visible region of the spectrum, and a higher Wavelength/m 10 10 103 10 10 10* 10 10 10 10. 10 10'12 10 10-10 1 cm 1 dm 1 mm. 1 pma 1 m um 700 n 1 nn 420 n Radio Micro Far infrared Vacuum Near Ultraviolet infrared X-ray y-ray Cosmic wave ultraviolet rays Molecular Molecular Electronic Core-electron Nuclear rotation vibration excitation excitation excitation Fig. 8.2 The electromagnetic spectrum and the classification of the spectral regions. 8.1 THE FAILURES OF CLASSICAL PHYSICS 245 proportion of short-wavelength blue light is generated as the temperature is raised. Maximum This behaviour is seen when a heated iron bar glowing red hot becomes white hot of p when heated further. The dependence is illustrated in Fig. 8.3, which shows how the energy output varies with wavelength at several temperatures. The curves are those of an ideal emitter called a black body, which is an object capable of emitting and absorbing all frequencies of radiation uniformly. A good approximation to a black body is a pinhole in an empty container maintained at a constant temperature, because any radiation leaking out of the hole has been absorbed and re-emitted inside Energy distribution, Increasing so many times that it has come to thermal equilibrium with the walls (Fig. 8.4). temperature The explanation of black-body radiation was a major challenge for nineteenth- century scientists, and in due course it was found to be beyond the capabilities of classical physics. The physicist Lord Rayleigh studied it theoretically from a classical viewpoint, and thought of the electromagnetic field as a collection of oscillators of all possible frequencies. He regarded the presence of radiation of frequency v (and therefore of wavelength 2 = c/v) as signifying that the electromagnetic oscillator of that frequency had been excited (Fig. 8.5). Rayleigh used the equipartition principle Wavelength, 2 (Section 2.2) to calculate the average energy of each oscillator as kT. Then, with minor help from James Jeans, he arrived at the Rayleigh-Jeans law (see Further reading for Fig. B.3 The energy distribution in a black- its justification): body cavity at several temperatures. Note how the energy density increases in the BackT region of shorter wavelengths as the dE= pd2 24 (8.3) temperature is raised, and how the peak shifts to shorter wavelengths. The total where p (rho), the density of states, is the proportionality constant between da energy density (the area under the curve) and the energy density, d'E, in the range of wavelengths between 2 and 2 + d2, k is increases as the temperature is increased Boltzmann's constant (k= 1.381 x 10-2 J K !). The units of p are typically joules per (as T*). metre* (] m *), to give an energy density d'E in joules per cubic metre (] m ) when multiplied by a wavelength range da in metres. A high density of states at the wave- length A simply means that there is a lot of energy associated with wavelengths lying between 2 and 2+ da. The total energy density (in joules per cubic metre) in a region is obtained by integrating eqn 8.3 over all wavelengths between zero and infinity, and the total energy (in joules) within the region is obtained by multiplying that total energy density by the volume of the region. Detected radiation Pinhole Container at a temperature T Fig. 8.5 The electromagnetic vacuum can be Fig. 8.4 An experimental representation of a regarded as able to support oscillations black-body is a pinhole in an otherwise of the electromagnetic field. When a high closed container. The radiation is reflected frequency, short wavelength oscillator many times within the container and (a) is excited, that frequency of radiation comes to thermal equilibrium with the is present. The presence of low frequency, walls at a temperature T. Radiation leaking long wavelength radiation (b) signifies out through the pinhole is characteristic that an oscillator of the corresponding of the radiation within the container. frequency has been excited.246 8 QUANTUM THEORY: INTRODUCTION AND PRINCIPLES Unfortunately (for Rayleigh, Jeans, and classical physics), although the Rayleigh- Rayleigh- Jeans law is quite successful at long wavelengths (low frequencies), it fails badly at Jeans law short wavelengths (high frequencies). Thus, as 2. decreases, p increases without going through a maximum (Fig. 8.6). The equation therefore predicts that oscillators of very Experimental short wavelength (corresponding to ultraviolet radiation, X-rays, and even y-rays) are strongly excited even at room temperature. This absurd result, which implies that a Energy density, large amount of energy is radiated in the high-frequency region of the electromag netic spectrum, is called the ultraviolet catastrophe. According to classical physics, even cool objects should radiate in the visible and ultraviolet regions, so objects should glow in the dark; there should in fact be no darkness. (b) The Planck distribution Wavelength, a The German physicist Max Planck studied black-body radiation from the viewpoint of thermodynamics. In 1900 he found that he could account for the experimental Fig. 8.6 The Rayleigh-Jeans law (eqn 8.3) predicts an infinite energy density at short observations by proposing that the energy of each electromagnetic oscillator is limited wavelengths. This approach to infinity is to discrete values and cannot be varied arbitrarily. This proposal is quite contrary called the ultraviolet catastrophe to the viewpoint of classical physics (on which the equipartition principle used by Rayleigh is based), in which all possible energies are allowed. The limitation of energies to discrete values is called the quantization of energy. In particular, Planck found that he could account for the observed distribution of energy if he supposed 251 that the permitted energies of an electromagnetic oscillator of frequency v are integer multiples of hv: E =nhv 1=0, 1, 2, ... (8.4) 20 where h is a fundamental constant now known as Planck's constant. On the basis of this assumption, Planck was able to derive the Planck distribution: 15 87the d'E= pda P=- (8.5) 25(ehdat - 1) PR8:(KT)'/(hc)) 8 (For references to the derivation of this expression, see Further reading.) This expression fits the experimental curve very well at all wavelengths (Fig. 8.7), and the value of h, which is an undetermined parameter in the theory, may be obtained by varying its value until a best fit is obtained. The currently accepted value for h is 6.626 x 10 34 J s. The Planck distribution resembles the Rayleigh-Jeans law (eqn 8.3) apart from the all-important exponential factor in the denominator. For short wavelengths, hcakT 0.5 1.0 1.5 2.0 > I and ehchair ->co faster than 25-> 0; therefore p-> 0as )-> 0or v->co. Hence, the akTinc energy density approaches zero at high frequencies, in agreement with observation. For long wavelengths, hodakT > 6) the exponentials in f can be expanded as 1+0,/T+ . . . and higher terms ignored (see Comment 8.2). The result is 1+0 /2T +... (+8, /T+...)-1 (8.8a) CA/R Consequently, the classical result (Cym =3R) is obtained at high temperatures. At low temperatures, when T 0 as T -> 0, and the heat capacity therefore approaches zero too. We see that Einstein's formula accounts for the decrease of heat capacity at low Fig. B.B Experimental low-temperature temperatures. The physical reason for this success is that at low temperatures only a molar heat capacities and the temperature few oscillators possess enough energy to oscillate significantly. At higher tempera- dependence predicted on the basis of tures, there is enough energy available for all the oscillators to become active: all 3N Einstein's theory. His equation (eqn 8.7) oscillators contribute, and the heat capacity approaches its classical value. accounts for the dependence fairly well, Figure 8.8 shows the temperature dependence of the heat capacity predicted by the but is everywhere too low. Einstein formula. The general shape of the curve is satisfactory, but the numerical Ex Exploration Using eqn 8.7, plot agreement is in fact quite poor. The poor fit arises from Einstein's assumption that all Gym against T for several values of the Einstein temperature . At low the atoms oscillate with the same frequency, whereas in fact they oscillate over a range temperature, does an increase in Oz result of frequencies from zero up to a maximum value, Vp- This complication is taken into in an increase or decrease of Cy? Estimate account by averaging over all the frequencies present, the final result being the Debye the temperature at which the value of CV.m formula: reaches the classical value given by eqn 8.6. Cvm= 3Rf 1=3 80). Te- 12 dx (8.9) where Op = hvp/k is the Debye temperature (for a derivation, see Further reading). The integral in eqn 8.9 has to be evaluated numerically, but that is simple with mathematical software. The details of this modification, which, as Fig. 8.9 shows, gives improved agreement with experiment, need not distract us at this stage from the main conclusion, which is that quantization must be introduced in order to explain the thermal properties of solids. Illustration 8.1 Assessing the heat capacity The Debye temperature for lead is 105 K, corresponding to a vibrational frequency of 2.2 x 1012 Hz, whereas that for diamond and its much lighter, more rigidly bonded atoms, is 2230 K, corresponding to 4.6 X 10" Hz. As we see from Fig. 8.9, (= 1 for T'> 0p and the heat capacity is almost classical. For lead at 25"C, corres- ponding to T/0) = 2.8, f=0.99 and the heat capacity has almost its classical value. For diamond at the same temperature, T/6 =0.13, corresponding to f=0.15, and the heat capacity is only 15 per cent of its classical value. (d) Atomic and molecular spectra The most compelling evidence for the quantization of energy comes from spec- troscopy, the detection and analysis of the electromagnetic radiation absorbed, emitted, or scattered by a substance. The record of the intensity of light transmitted 8.2 WAVE-PARTICLE DUALITY 249 Deby Einstein N Absorption intensity Emission intensity 200 240 280 320 Wnm 0.5 T10, or TWO. Fig. 8.11 When a molecule changes its state, Fig. 8.9 Debye's modification of Einstein's it does so by absorbing radiation at definite calculation (eqn 8.9) gives very good 415 420 frequencies. This spectrum is part of that agreement with experiment. For copper, Wavelength, Wm due to the electronic, vibrational, and T/0 = 2 corresponds to about 170 K, so rotational excitation of sulfur dioxide the detection of deviations from Dulong Fig. 8.10 A region of the spectrum of (SO,) molecules. This observation suggests and Petit's law had to await advances in radiation emitted by excited iron atoms that molecules can possess only discrete low-temperature physics. consists of radiation at a series of discrete energies, not an arbitrary energy. Ly Exploration Starting with the Debye wavelengths (or frequencies). He formula (eqn 8.9), plot dCy, /dT, the temperature coefficient of Cy,, against Tfor 60 = 400 K. At what temperature is Cym most sensitive to temperature? or scattered by a molecule as a function of frequency (v), wavelength (2), or wavenumber (v= w/c) is called its spectrum (from the Latin word for appearance). A typical atomic spectrum is shown in Fig. 8.10, and a typical molecular spectrum is shown in Fig. 8.11. The obvious feature of both is that radiation is emitted or absorbed at a series of discrete frequencies. This observation can be understood if the energy of the atoms or molecules is also confined to discrete values, for then energy can be dis- carded or absorbed only in discrete amounts (Fig. 8.12). Then, if the energy of an atom Energy decreases by AE, the energy is carried away as radiation of frequency v, and an emission hy= E, -E line', a sharply defined peak, appears in the spectrum. We say that a molecule undergoes spectroscopic transition, a change of state, when the Bohr frequency condition Iv= E -E MAAMA AE = hv (8.10 is fulfilled. We develop the principles and applications of atomic spectroscopy in Chapter 10 and of molecular spectroscopy in Chapters 13-15. Fig. 8.12 Spectroscopic transitions, such as 8.2 Wave-particle duality those shown above, can be accounted for if we assume that a molecule emits a photon At this stage we have established that the energies of the electromagnetic field and of as it changes between discrete energy levels. oscillating atoms are quantized. In this section we shall see the experimental evidence Note that high-frequency radiation is that led to the revision of two other basic concepts concerning natural phenomena. emitted when the energy change is large. One experiment shows that electromagnetic radiation-which classical physics treats as wave-like-actually also displays the characteristics of particles. Another experi- ment shows that electrons-which classical physics treats as particles-also display the characteristics of waves.250 8 QUANTUM THEORY: INTRODUCTION AND PRINCIPLES (a) The particle character of electromagnetic radiation The observation that electromagnetic radiation of frequency v can possess only the energies 0, hv, 2hv, . . . suggests that it can be thought of as consisting of 0, 1, 2, ... particles, each particle having an energy hv. Then, if one of these particles is present, the energy is hy, if two are present the energy is 2hv, and so on. These particles of electromagnetic radiation are now called photons. The observation of discrete spectra from atoms and molecules can be pictured as the atom or molecule generating a photon of energy hy when it discards an energy of magnitude AE, with AE = hv. Example 8.1 Calculating the number of photons Calculate the number of photons emitted by a 100 W yellow lamp in 1.0 s. Take the wavelength of yellow light as 560 nm and assume 100 per cent efficiency. Method Each photon has an energy hy, so the total number of photons needed to produce an energy E is E/hv. To use this equation, we need to know the frequency of the radiation (from v= /)) and the total energy emitted by the lamp. The latter is given by the product of the power (P, in watts) and the time interval for which the lamp is turned on (E= PAt). Answer The number of photons is N=- E PAt APAL hy h(edA) he Substitution of the data gives N=- (5.60 x 10-7 m) x (100 ] s") x (1.0s) =2.8x 1020 (6.626 x 10 J s) x (2.998 x 10 ms ) Note that it would take nearly 40 min to produce 1 mol of these photons. A note on good practice To avoid rounding and other numerical errors, it is best to carry out algebraic manipulations first, and to substitute numerical values into a single, final formula. Moreover, an analytical result may be used for other data without having to repeat the entire calculation. Self-test 8.1 How many photons does a monochromatic (single frequency) infrared rangefinder of power 1 mW and wavelength 1000 nm emit in 0.1 s? [5x 10'4] Further evidence for the particle-like character of radiation comes from the meas- urement of the energies of electrons produced in the photoelectric effect. This effect is the ejection of electrons from metals when they are exposed to ultraviolet radiation. The experimental characteristics of the photoelectric effect are as follows: 1 No electrons are ejected, regardless of the intensity of the radiation, unless its frequency exceeds a threshold value characteristic of the metal. 2 The kinetic energy of the ejected electrons increases linearly with the frequency of the incident radiation but is independent of the intensity of the radiation. 3 Even at low light intensities, electrons are ejected immediately if the frequency is above the threshold. 8.2 WAVE-PARTICLE DUALITY 251 Kinetic energy of ejected electron Energy needed to remove imv 2.30 eV (1.86 x 10' cm ', 539 nm) 2.25 eV (1.81 x 10' cm ', 551 nm) 2.09 ev (1.69 x 10' cm ', 593 nm) electron from Rubidium metal Potassium Sodium Kinetic energy of photoelectron, Ex Increasing work function Frequency of incident (a) (b) radiation, v Fig. 8.13 In the photoelectric effect, it is Fig. 8.14 The photoelectric effect can be found that no electrons are ejected when explained if it is supposed that the incident the incident radiation has a frequency radiation is composed of photons that have below a value characteristic of the metal energy proportional to the frequency of the and, above that value, the kinetic energy of radiation. (a) The energy of the photon is the photoelectrons varies linearly with the insufficient to drive an electron out of the frequency of the incident radiation. metal. (b) The energy of the photon is Exploration Calculate the value of more than enough to eject an electron, Planck's constant given that the and the excess energy is carried away as the kinetic energy of the photoelectron following kinetic energies were observed (the ejected electron). or photoejected electrons irradiated by radiation of the wavelengths noted. Am 320 330 345 360 385 ExleV 1.17 1.05 0.885 0.735 0.511 Figure 8.13 illustrates the first and second characteristics. These observations strongly suggest that the photoelectric effect depends on the ejection of an electron when it is involved in a collision with a particle-like projectile that carries enough energy to eject the electron from the metal. If we suppose that the projectile is a photon of energy hy, where v is the frequency of the radiation, then the conservation of energy requires that the kinetic energy of the ejected electron should obey mul=hv-@ (8.11) In this expression
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


