a. i. Explain why some substances are radioactive and some are not. ii. State the cause of
Question:
a. i. Explain why some substances are radioactive and some are not.
ii. State the cause of background radiation.
iii. Explain what you understand by the meaning of the half-life of a radioactive element.
b. Technetium-99m is a radioactive material with a half-life of 6 hours. It is used to study blood flow around the body. A sample of technetium-99m has an activity of 96 counts per minute when injected into a patient's blood stream. Estimate
i. Its activity after 12 hours
ii. How long it will take for the radioactivity from the injection to become undetectable.
c. Technetium-99m is a gamma (γ) emitter and does not produce alpha (α) or beta (β) radiations. Explain why it is safe to inject technetium-99m into the body.
d. Radioactive salt (sodium chloride) is also used in medicine. The radioactive sodium (Na) in the salt decays, according to the equation shown below, to form magnesium (Mg).
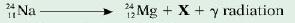
i. Name the particle X.
ii. Use the information given in the equation above to find the
I. Total number of charged particles in each sodium atom.
II. number of neutrons in the nucleus of a sodium 24 atom.
Step by Step Answer:






