A compound with molecular formula C 5 H 10 O 2 has the following NMR spectrum. Determine
Question:
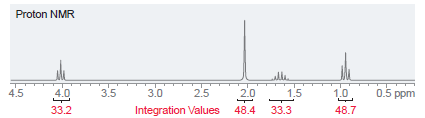
Transcribed Image Text:
Proton NMR lle 2.0 1.5 0.5 ppm 4.5 4.0 33.2 3.5 3.0 2.5 1.0 Integration Values 48.4 33.3 48.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (19 reviews)
The signal at 40 ppm represents two ...View the full answer

Answered By

Muhammad Khurram
I have strong General Management skills to apply in your projects. Over last 3 years, I have acquired great knowledge of Accounting, Auditing, Microsoft Excel, Microsoft PowerPoint, Finance, Microsoft Project, Taxation, Strategic Management, Human Resource, Financial Planning, Business Planning, Microsoft Word, International Business, Entrepreneurship, General Management, Business Mathematics, Advertising, Marketing, Supply Chain, and E-commerce. I can guarantee professional services with accuracy.
4.80+
249+ Reviews
407+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound with molecular formula C4H8O has a strong IR absorption at 1730 cm-1. Its mass spectrum includes key peaks at m/z 44 (the base peak) and m/z 29. Propose a structure for the compound and...
-
A compound with molecular formula C4H6O gives the infrared spectrum shown in Figure 13.34. Identify the compound. 3.5 4 5 12 13
-
When a compound with molecular formula C11H14O2 undergoes acid-catalyzed hydrolysis, one of the products that is isolated gives the following 1H NMR spectrum. Identify the compound. 10 9 4
-
Jay Bhattacharya and Kate Bundorf of Stanford University have found evidence that people who are obese and who work for firms that provide health insurance receive lower wages than workers at those...
-
Using the loanable funds market diagram, demonstrate the effects of the following: A. A decrease in the saving rate B. An increase in business investment spending C. A reduction in the federal...
-
Larry Jones and his co-worker were on shift as security guards in a large warehouse. They heard noise coming from a far corner of the building and went to investigate. At that point, three men jumped...
-
From the following particulars, prepare a Bank Reconciliation Statement as on 31 December 1999: (a) Bank overdraft as per passbook 26,000 (b) A cheque for (c) Cheques for were presented 3000 sent for...
-
What moral, ethical, and professional responsibilities did Guy Enright face when he was asked to turn over confidential documents to the individuals who were representing themselves as intelligence...
-
Question 2 [20 points] The account balances for the noncash current assets and current liabilities of Stake Technology Inc. are as follows : Question 2 [20 points) The account balances for the...
-
Consider each of the following arguments. If the argument is valid, identify the rule of inference that establishes its validity. If not, indicate whether the error is due to an attempt to argue by...
-
For each of the following compounds, identify the expected chemical shift for each type of proton: (a) (b) (c) (d)
-
A compound with molecular formula C 10 H 10 O has the following NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 10 3 1 ppm 4 87.1| 18.7 Integration Values 17.1...
-
A +2.0 nC charge is at the origin and a -4.0 nC charge is at x = 1.0 cm. a. At what x-coordinate could you place a proton so that it would experience no net force? b. Would the net force be zero for...
-
The following information was obtained from the records of Shae Inc.: Merchandise inventory $ 88,000 Notes payable (long-term) 100,000 Net sales 300,000 Buildings and equipment 168,000 Selling,...
-
Absent Clothing Company Savita Kapur, CEO, founded Absent Clothing Company (ACC) in 2005. ACC sells practical athletic wear to service the yoga and pilates market. Savita originally created ACC with...
-
Find the indicated area under the curve of the standard normal distribution; then convert it to a percentage and fill in the blank. About % of the area is between z = - 3.5 and z = 3.5 (or...
-
EM 605 Spring 2021 Midterm Exam 3/17/2021 The linear programming problem whose output follows is used to determine how many bottles of Hell-bound red nail polish (x1), Blood red nail polish (x2),...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Sketch a graph of y = f(x). x = (x)f
-
Illini Company, Inc. Balance Sheet as of 12/31/20X0 Assets Current Assets: Cash $1,500,000 Accounts receivable, net 18,000 Inventory 50,000 Total current assets 1,568,000 Equipment 90,000 Goodwill...
-
Increased substitution around a bond leads to increased strain. Take the four substituted butanes listed below, for example. For each compound, sight along the C2-C3 bond and draw Newman projections...
-
The cholesterol-lowering agents called statins, such as simvastatin (Zocor) and pravastatin (Pravachol), are among the most widely prescribed drugs in the world. Identify the functional groups in...
-
We?ll look at cycloalkanes?saturated cyclic hydrocarbons? and we?ll see that the molecules generally adopt puckered, non-planar conformations. Cyclohexanc, for instance, has a puckered shape like a...
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


