We?ll look at cycloalkanes?saturated cyclic hydrocarbons? and we?ll see that the molecules generally adopt puckered, non-planar conformations.
Question:
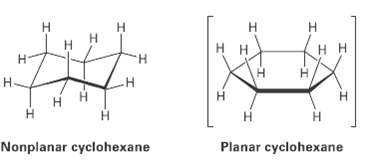
We?ll look at cycloalkanes?saturated cyclic hydrocarbons? and we?ll see that the molecules generally adopt puckered, non-planar conformations. Cyclohexanc, for instance, has a puckered shape like a lounge chair rather than a flat shape. Why?
Transcribed Image Text:
Н н H. H. H. H H Н H. Н- Н H. Н Planar cyclohexane Nonplanar cyclohexane I-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
A puckered ring allows all the ...View the full answer

Answered By

Shubham gupta
This is Swetha. I have completed Bachelors degree in 2016 and my background is Electronics and communication Engineering.
I have four years experience in tutoring.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Why do we focus on cash flows rather than accounting profits in making our capital- budgeting decisions? Why are we interested only in incremental cash flows rather than total cash flows?
-
Why do economists generally favor vouchers rather than public production or subsidies to achieve an efficient outcome?
-
Why is automation generally regarded as evolutionary rather than revolutionary? Explain.
-
Consider examples 9.2 and 9.3. Assume that social damage is quadratic as in the examples. Following section 9.2.3, assume that the regulator can only set a uniform tax. Determine the formula for the...
-
In the prospectus for the Brazos Small Cap Fund, the fee table indicates that the fund has a 12b -1 fee of 0.35 percent and an expense ratio of 1.65 percent that is collected once a year on December...
-
What is the decision facing Airbus?
-
Matt Albin, Ryan Peters, and Seth Ramsey invested $164,000, $98,400, and $65,600, respectively, in a partnership. During its first calendar-year, the firm earned $270,000. Required Prepare the entry...
-
On March 1, 2015, Zephur Winds Ltd. purchased a machine for $80,000 by paying $20,000 down and issuing a note for the balance. The machine had an estimated useful life of nine years and an estimated...
-
a. Use the appropriate formula to find the value of the annuity. b. Find the interest
-
A gas containing only CH4 and N2 is burned with air yielding a flue gas that has an Orsat analysis of CO2 8.7%, CO 1.0%, O2 3.8%, and N2 86.5%. Calculate the percent excess air used in combustion and...
-
The cholesterol-lowering agents called statins, such as simvastatin (Zocor) and pravastatin (Pravachol), are among the most widely prescribed drugs in the world. Identify the functional groups in...
-
We?ll see that there are two isomeric substances both named 1, 2-dimethylcyclohexane. Explain. -C3 1,2-Dimethylcyclohexane CH
-
Colorado Corporation has two classes of stock: common, $3 par value; and preferred, $30 par value. Requirements 1. Journalize Colorado's issuance of 4,500 shares of common stock for $6 per share. 2....
-
What is an incident in which a famous person wore or used a product (not as part of a paid endorsement or ad) and it caused a buying frenzy. Explain how the manufacturer or service provider reacted
-
What is a "heavyweight project team" and how does it differ from the traditional approach used for organizing development projects at Eli Lilly?This consists of two issues:First, an evaluation of the...
-
Consider the closed-loop system shown in Figure P11.6, where the transfer function of the process is that of a second-order system, i.e. k Ts +25TS +1 G,(s)= Y sp(s) E(s) U(s) Y(s) Ge(s) Gp(s) Figure...
-
1. Do you feel we have come along way with inventory in 10 years? 2. How did COVID affect the supply chain in your current hospital? Were any of the inventory systems/topics used, or relevant or...
-
Identify at least one way in which your writing skills have improved this semester and reflect on how you might use this skill in your career. You can include research, presentation, and report...
-
view the writing of the final project report as an exciting prospect. LO3
-
Linda Lopez opened a beauty studio, Lindas Salon, on January 2, 2011. The salon also sells beauty supplies. In January 2012, Lopez realized she had never filed any tax reports for her business and...
-
With reference to Figure 12-20, which is the more volatile liquid, benzene or toluene? At approximately what temperature does the less volatile liquid have the same vapor pressure as the more...
-
Starting with the compound or compounds indicated in each part and using any other needed reagents, outline syntheses of each of the following compounds. (You need not repeat steps carried out in...
-
How would the molecular ion peaks in the respective mass spectra of CH3Cl, CH2 Cl2, CHCl3, and CCl4 differ on the basis of the number of chlorines? (Remember that chlorine has isotopes 35Cl and 37Cl...
-
Provide the reagents necessary for the following synthetic transformations. More than one step may be required. (a) (b) (c) (d) (e) (f) OCH3 Br
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


