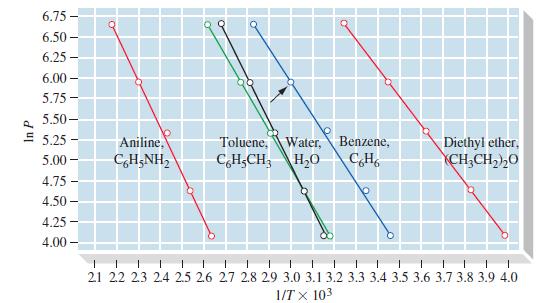
With reference to Figure 12-20, which is the more volatile liquid, benzene or toluene? At approximately what
Question:
With reference to Figure 12-20, which is the more volatile liquid, benzene or toluene? At approximately what temperature does the less volatile liquid have the same vapor pressure as the more volatile one at 65 °C?
Figure 12-20
Transcribed Image Text:
6.75 - 6.50- 6.25- 6.00- 5.75 - 5.50- 5.25 5.00- 4.75 - 4.50- 4.25- 4.00- Aniline, CHẠNH, O Toluene, Water, C6H-CH3 H₂O Benzene, C6H6 Diethyl ether, CH₂CH₂)20 1 1 1 I 1 I 1 1 1 I I 1 I 1 1 I I 1 1 21 22 23 24 25 26 27 28 29 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4.0 1/TX 103
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The more volatile liquid in Figure 1220 is benzene Volatility is the tendency of a liquid to evap...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Selected information from Indigo Books & Music Inc.'s income statements for three recent years follows (dollars in thousands): Instructions (a) Calculate gross profit, and profit from operations, for...
-
Guarino and two others (plaintiffs) died of gas asphyxiation and five others were injured when they entered a sewer tunnel without masks to answer the cries for help of their crew leader, Rooney....
-
What fund types should be used for a government university engaged in both governmental and business-type activities?
-
Arrange to visit an attraction or entertainment location in your area and schedule an interview with the manager or local administrator. Your interview should include questions about the typical...
-
Horizontal analysis (trend analysis) percentages for Epstein Companys sales, cost of goods sold, and expenses are shown below. Did Epsteins net income increase, decrease, or remain unchanged over the...
-
I need the adjusting entries to be completed correctly. Also Any other associated content in regards to this question would be much appretiated ADJUSTMENT DATA ENTRY BELOW: On November 1, 2022, the...
-
Data table Buckland Associates Bank Reconciliation Cash February 28, 2025 Bank: Beg. Bal 4,995 650 Feb. 3 Balance, February 28, 2025 4.707 Feb 6 600 2200 Feb. 12 Add Deposit in transit 2.200 Feb. 15...
-
The vapor pressure of trichloromethane (chloroform) is 40.0 Torr at -7.1 C. Its enthalpy of vaporization is 29.2 kJ mol -1 . Calculate its normal boiling point.
-
The normal boiling point of acetone, an important laboratory and industrial solvent, is 56.2 C and its vap H is 25.5 kJ mol -1 . At what temperature does acetone have a vapor pressure of 375 mmHg?
-
Search online for two examples of violations of OSHA regulations in each of these categories: serious, willful, and repeated. Explain the violation, the fine, or any other penalties involved. Look...
-
Required information [The following information applies to the questions displayed below.] The following is financial information describing the six operating segments that make up Chucktown Sauce...
-
Question 1 (50 marks) Costa Ltd is a company with a 30 June year end. The following information relates to Costa Ltd and its subsidiary Jumbo for the year ended 30 June 20.22. Costa Ltd Jumbo Ltd Dr...
-
The following salaried employees of Mountain Stone Brewery in Fort Collins, Colorado, are paid semimonthly. Some employees have union dues or garnishments deducted from their pay. You do not need to...
-
Dr. Burgess oversees the pharmacy center within Hughes Regional Hospital. Dr. Burgess is planning on purchasing two medication dispensing units which she wants to pay back in a short-term period. The...
-
On January 1, 2021, Wetick Optometrists leased diagnostic equipment from Southern Corp., which had purchased the equipment at a cost of $1,831,401. The lease agreement specifies six annual payments...
-
Methyl orange is an azo dye used as an indicator in acid- base titrations. (It is yellow-orange above pH 4.5 and red below pH 3.) Show how it can be synthesized from p aminobenzenesulfonic acid...
-
Element compound homogeneous mixture (heterogeneous mixture) 4) A piece of gold has a mass of 49.75 g. What should the volume be if it is pure gold? Gold has a density of 19.3 g/cm (3 points) D=m/v...
-
Zero Coupon Bonds suppose your company needs to raise $20 million and you want to issue 30-year bonds for this purpose. Assume the required return on your bond issue will be 7 percent, and youre...
-
Finding the Maturity youve just found a 10 percent coupon bond on the market that sells for par value. What is the maturity on this bond?
-
Real Cash Flows you want to have $1 million in real dollars in an account when you retire in 40 years. The nominal return on your investment is 11 percent and the inflation rate is 4.5 percent. What...
-
1) issued stock for $72,000 2) borrowed $41,000 from its bank 3) provided consulting services for $71,000 cash 4) paid back $31,000 of the bank loan 5) paid rent expense for $17,000 6) purchased...
-
Centurion Co. had the following accounts and balances at December 31: Account Cash Accounts Receivable Prepaid Insurance Supplies Accounts Payable T. Happy, Capital Service Revenue Salaries Expense...
-
Gretchen invests 6200 dollars in a mutual fund on January 1. On March 1, she learns that her fund balance is 3800 dollars, and she then withdraws 1500 dollars. On August 1, her fund balance is 7800...

Study smarter with the SolutionInn App


