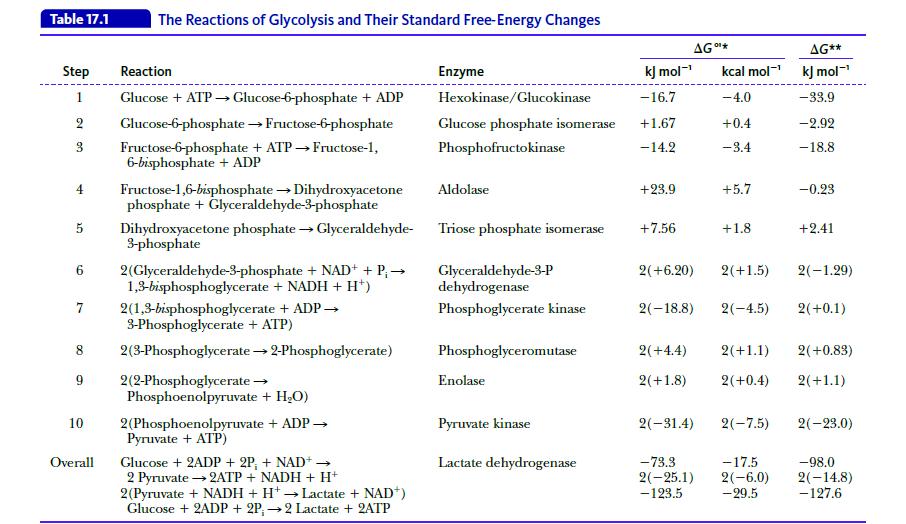
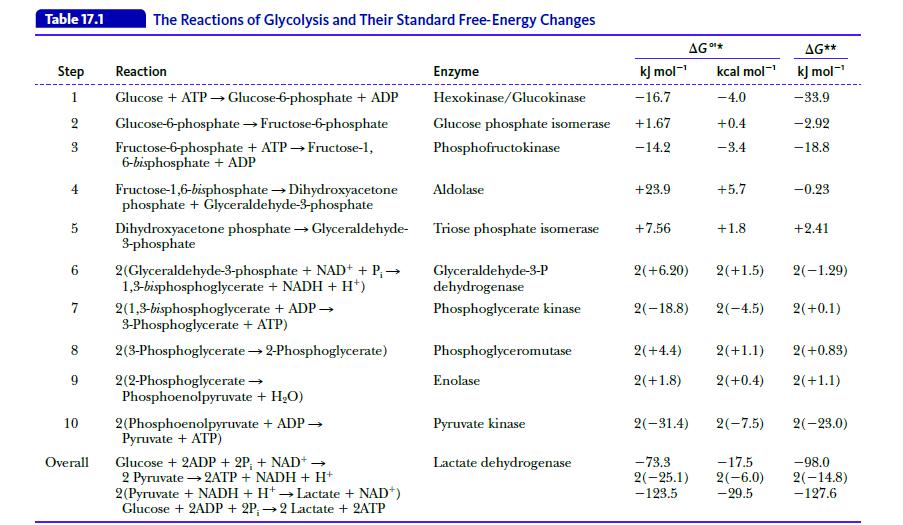
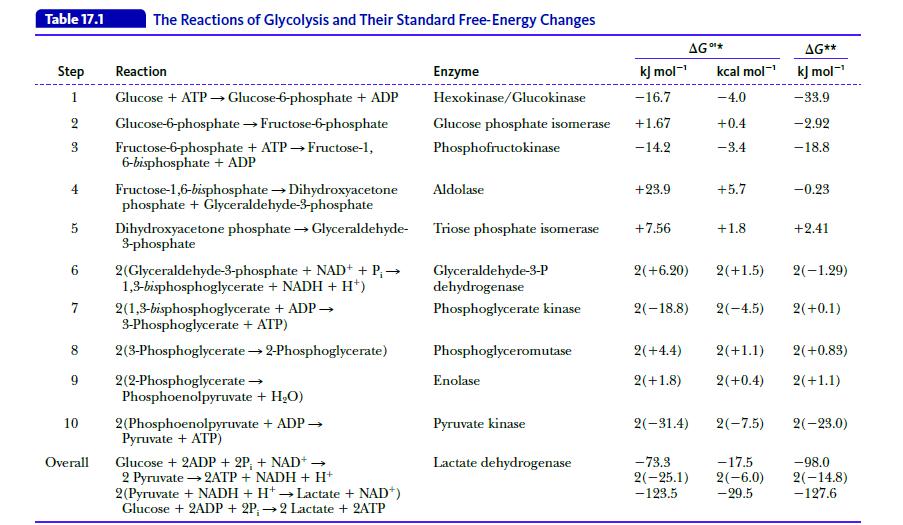
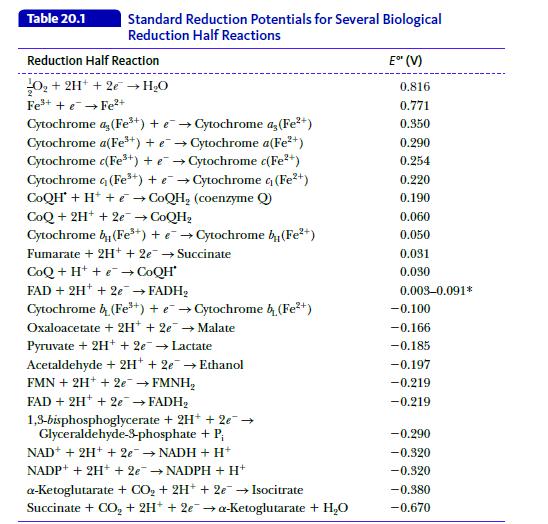
Biochemistry 9th Edition Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal - Solutions
Unlock the full potential of "Biochemistry 9th Edition" by Mary K. Campbell, Shawn O. Farrell, and Owen M. McDougal with our comprehensive solutions. Access a wealth of resources, including an answers key and a detailed solution manual available as a solutions PDF. Discover expertly solved problems, complete with step-by-step answers, to enhance your understanding. Our test bank and instructor manual offer an array of questions and answers for effective study. Explore chapter solutions and the entire textbook content for free download online, providing a robust foundation for mastering biochemistry concepts.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()