Show that the reaction Glucose 2 Glyceraldehyde-3-phosphate is slightly endergonic (G' = 2.2 kJ mol -1
Question:
Show that the reaction Glucose → 2 Glyceraldehyde-3-phosphate is slightly endergonic (ΔG°' = 2.2 kJ mol-1 = 0.53 kcal mol-1); that is, it is not too far from equilibrium. Use the data in Table 17.1.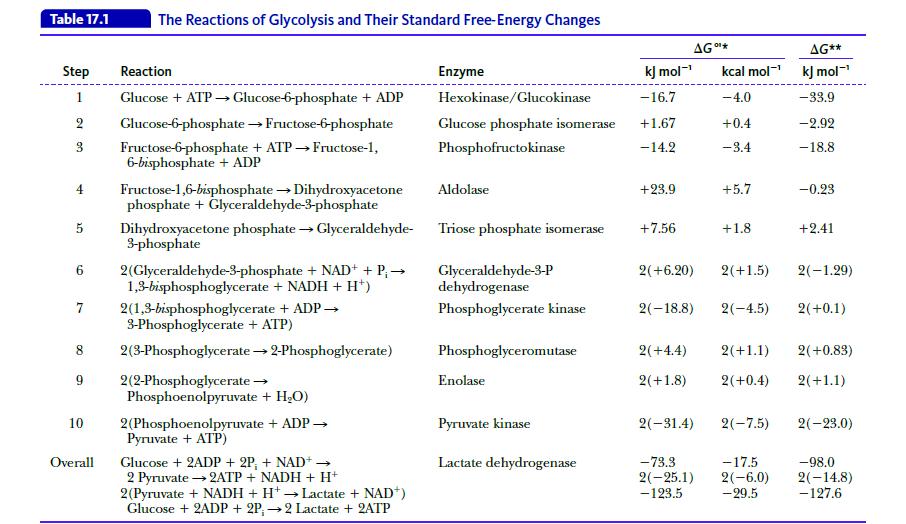
Transcribed Image Text:
Table 17.1 Step Reaction 1 Glucose + ATP → Glucose-6-phosphate + ADP 2 Glucose-6-phosphate → Fructose-6-phosphate 3 Fructose-6-phosphate + ATP→Fructose-1, 6-bisphosphate + ADP 4 Fructose-1,6-bisphosphate → Dihydroxyacetone phosphate + Glyceraldehyde-3-phosphate 5 Dihydroxyacetone phosphate → Glyceraldehyde- 3-phosphate 6 2(Glyceraldehyde-3-phosphate + NAD+ + P₁ → 1,3-bisphosphoglycerate + NADH + H¹) 7 The Reactions of Glycolysis and Their Standard Free-Energy Changes 8 9 10 Overall 2(1,3-bisphosphoglycerate + ADP → 3-Phosphoglycerate + ATP) 2(3-Phosphoglycerate → 2-Phosphoglycerate) 2(2-Phosphoglycerate → Phosphoenolpyruvate + H₂O) 2(Phosphoenolpyruvate + ADP → Pyruvate + ATP) Glucose + 2ADP + 2P, + NAD+ → 2 Pyruvate → 2ATP + NADH + H+ - 2(Pyruvate + NADH + H+ → Lactate + NAD+) Glucose + 2ADP + 2P;→2 Lactate + 2ATP Enzyme kJ mol-¹ Hexokinase/Glucokinase -16.7 Glucose phosphate isomerase +1.67 Phosphofructokinase -14.2 Aldolase Triose phosphate isomerase Glyceraldehyde-3-P dehydrogenase Phosphoglycerate kinase Phosphoglyceromutase Enolase Pyruvate kinase Lactate dehydrogenase +23.9 AG *** +7.56 kcal mol-¹ -4.0 +0.4 -3.4 2(-18.8) +5.7 +1.8 2(+6.20) 2(+1.5) 2(-4.5) 2(+4.4) 2(+1.8) 2(+0.4) 2(-31.4) 2(-7.5) AG** kJ mol-¹ -33.9 -2.92 -18.8 -73.3 -17.5 2(−25.1) 2(-6.0) -123.5 - 29.5 -0.23 +2.41 2(+1.1) 2(+0.83) 2(+1.1) 2(-1.29) 2(+0.1) 2(-23.0) -98.0 2(-14.8) -127.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Add the G mol 1 values f...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
For the reaction N 2 (g) + 3 H 2 (g) 2 NH 3 (g), the equilibrium constant is K p = 36.5 at 400 K. Two separate equilibrium mixtures have the following compositions at 400 K and a total pressure of...
-
The first nuclear reaction ever observed (in 1919 by Ernest Rutherford) was a + 147N p+ X. (a) Show that the reaction product "X" must be 178O. (b) For this reaction to take place, the particle...
-
Why program planning is important in public health leadership?
-
The Cutting Department of Hong Manufacturing has the following production and cost data for July. Instructions(a) Determine the equivalent units of production for (1) Materials and (2) Conversion...
-
Suppose you want to approximate 3 128 to within 10 -4 of the exact value. a. Use a Taylor polynomial for f(x) = (125 + x) 1/3 centered at 0. b. Use a Taylor polynomial for f(x) = x 1/3 centered at...
-
Explain the four market-product strategies in diversification analysis. LO.1
-
Study the following Minitab output from a regression analysis to predict y from x. a. What is the equation of the regression model? b. What is the meaning of the coefficient of x? c. What is the...
-
You are deciding whether to grant a customer credit for an order just received. The company's credit terms are net 60 days. The opportunity cost of funds is 15%. The order dollar amount is $50,000....
-
What are some of the main differences between the cell walls of plants and those of bacteria?
-
A friend asks you why some parents at her childs school want a choice of beverages served at lunch, rather than milk alone. What do you tell your friend?
-
The idea that Jakes mother is trying her best to take care of her son is least supported by which of the following quotations from the passage? A. Its just that I work back-to-back jobs every night...
-
6.10 Long Div. and Comp Square Calculus - No Calculator Find the indefinite integral. 1. S 4x-34x+56x-21 4x-2 dx Mastery Check #2 1 dx 2. Sx-4x+5x x-4x+5 S S Name: Sienna Nono Date: 3-1-24 Period:...
-
How well are oncology firms leveraging digital technology to gain and sustain competitive advantage?
-
Axel and Brooklyn have agreed to buy a new vehicle. Brooklyn mentions that she is looking forward to getting a new SUV, so they have room for their dogs and kids. Axel mentions he thought they were...
-
Discuss how technology and human resources are needed to operate this facility in this behind the scenes look at this retailing giant. Support your opinion with research and/or key concepts covered...
-
4.2 At a given instant, a spacecraft is 500 km above the earth, with a right ascension of 300 and a declination of -20 relative to the geocentric equatorial frame. Its velocity is 10 km/s directly...
-
(a) For CH2O, run HF/3-21G geometry optimization and vibrational-frequency calculations to obtain the predicted geometry, dipole moment, and harmonic vibrational wavenumbers. Verify that all...
-
According to a New York Times columnist, The estate tax affects a surprisingly small number of people. In 2003, . . . just 1.25 percent of all deaths resulted in taxable estates, with most of them...
-
A small DNA molecule was cleaved with several dierent restriction nucleases, and the size of each fragment was determined by gel electrophoresis. The following data were obtained. Enzyme Fragment...
-
Te average human chromosome contains about 1 108 bp of DNA. (a) If each base pair has a mass of about 660 daltons and there are about 2 g of protein (histones plus nonhistones) per gram of DNA, how...
-
Forming nucleosomes and wrapping them into a 30-nm ber provide part of the compaction of DNA in chromatin. If the ber contains about six nucleosomes per 10 nm of length, what is the approximate...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


