For the reaction N 2 (g) + 3 H 2 (g) 2 NH 3 (g), the
Question:
For the reaction N2(g) + 3 H2(g) ⇌ 2 NH3(g), the equilibrium constant is Kp = 36.5 at 400 K. Two separate equilibrium mixtures have the following compositions at 400 K and a total pressure of 1.00 bar.
Equilibrium mixture A:
0.0424 mol N2 0.136 mol H2 0.176 mol NH3
Equilibrium mixture B:
0.194 mol N2 0.0403 mol H2 0.0706 mol NH3
(a) By expressing the partial pressures in the form Pi = xiP, where xi is the mole fraction of a particular gas and P is the total pressure, show that the reaction quotient for the reaction can be written as
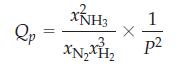
(b) Calculate Qp for each equilibrium mixture to verify that Qp = Kp in each case.
(c) Suppose that 0.100 mol N2 is added at constant pressure to each mixture. By comparing the values of Qp and Kp, verify that the addition of N2 causes a net reaction to the right for mixture Abut a net reaction to the left for mixture B.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





