Recall the formation of methanol from synthesis gas, the reversible reaction at the heart of a process
Question:
Recall the formation of methanol from synthesis gas, the reversible reaction at the heart of a process with great potential for the future production of automotive fuels.
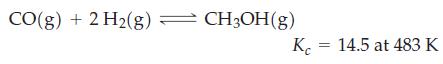
A particular synthesis gas consisting of 35.0 mole percent CO(g) and 65.0 mole percent H2(g) at a total pressure of 100.0 atm at 483 K is allowed to come to equilibrium. Determine the partial pressure of CH3OH(g) in the equilibrium mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





