Why are all the reactions in Table 20.1 written as reduction reactions? Table 20.1 Standard Reduction Potentials
Question:
Why are all the reactions in Table 20.1 written as reduction reactions?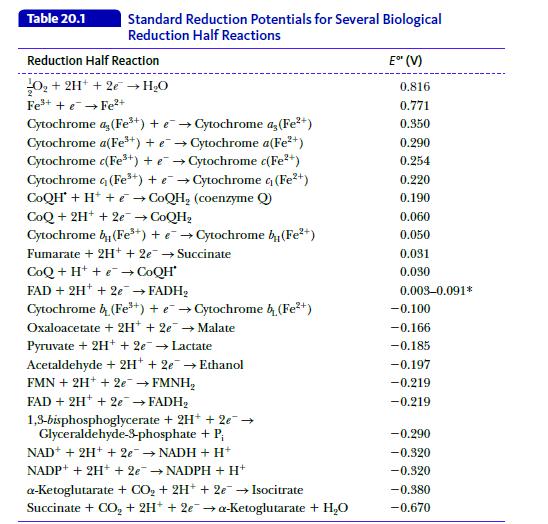
Transcribed Image Text:
Table 20.1 Standard Reduction Potentials for Several Biological Reduction Half Reactions Reduction Half Reaction O₂ + 2H+ + 2e → H₂O Fe³+ + Fe²+ Cytochrome a, (Fe³+) + e Cytochrome as (Fe²+) Cytochrome a(Fe³+) + e→Cytochrome a(Fe²+) Cytochrome c(Fe³+) + e→Cytochrome c(Fe²+) Cytochrome 4 (Fe³+) + e→Cytochrome 4 (Fe²+) CoQH + H+ e→CoQH₂ (coenzyme Q CoQ+ 2H+ + 2e → CoQH₂ Cytochrome by (Fe³+) + e→Cytochrome b(Fe²+) Fumarate + 2H+ + 2e → Succinate CoQ + H+ + e→CoQH* FAD + 2H+ + 2e → FADH₂ Cytochrome (Fe³+) + e→Cytochrome b (Fe²+) Oxaloacetate + 2H+ + 2e → Malate Pyruvate + 2H+ + 2e → Lactate Acetaldehyde + 2H+ 2e → Ethanol FMN + 2H+ + 2e → FMNH₂ FAD + 2H+ + 2e → FADH₂ 1,3-bisphosphoglycerate + 2H+ + 2e → Glyceraldehyde-3-phosphate + P₁ NAD+ + 2H+ + 2e → NADH + H+ NADP+ + 2H+ + 2e → NADPH + H+ a-Ketoglutarate + CO₂ + 2H+ + 2e → Isocitrate Succinate + CO₂ + 2H+ + 2e → a-Ketoglutarate + H₂O E" (V) 0.816 0.771 0.350 0.290 0.254 0.220 0.190 0.060 0.050 0.031 0.030 0.003-0.091* -0.100 -0.166 -0.185 -0.197 -0.219 -0.219 -0.290 -0.320 -0.320 -0.980 -0.670
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The reactions are all written ...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Write a literature review for your study. See below for an example of a literature review. Your literature review should provide both analysis and synthesis of previous studies as related to the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Define pros and cons of the current transportation strategy of BEST and elaborate recommendations.
-
The Polishing Department of Burgoa Manufacturing Company has the following production and manufacturing cost data for September. Materials are entered at the beginning of the process. Production:...
-
Find the function represented by the following series and find the interval of convergence of the series. (Not all these series are power series.) ,-kx k=0
-
What are examples of a functional level in an organization? LO.1
-
On January 1, 2010, the Easton Corporation acquired 30% of the outstanding common shares of Feeley Corporation for $140,000, and 25% of the outstanding common shares of Holmes Company for $82,500 and...
-
The following financial statements and additional information are reported. IKIBAN INCORPORATED Comparative Balance Sheets At June 3 0 2 0 2 1 2 0 2 0 Assets Cash $ 7 9 , 7 0 0 $ 5 7 , 0 0 0 Accounts...
-
Would you expect G' for the hydrolysis of a thioester to be (a) Large and negative, (b) Large and positive, (c) Small and negative, or (d) Small and positive? Give the reason for your answer.
-
According to Table 17.1, several reactions have very positive G' values. How can this be explained, given that these reactions do occur in the cell? Table 17.1 Step Reaction 1 Glucose + ATP ...
-
Confirm that the observed gas-phase structures of XeF 2 , XeF 4 and XeF 6 are consistent with the VSEPR model.
-
What are the elements of partnership ?
-
1. (2x+3)dx
-
For C4H9OH, give the number of CGTFs used in a calculation with each of the following basis sets: (a) STO-3G; (b) 3-21G; (c) 6-31G*; (d) 6-31G**; (e) 6-31+G*; (f) cc-pVTZ; (g) cc-pVQZ; (h)...
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
GTP g S is a nonhydrolyzable analog of GTP. Suppose this compound were added to a cell-free system containing active components of an adrenergic signaling system. What consequences would you expect?...
-
Suppose that you had a monoclonal antibody that recognized phosphotyrosine. How would you expect that antibody to aect insulin signaling?
-
Signaling molecules interact with cells through specic macromolecular receptors. For each of the four receptors identied below, list all characteris-tics, by number, which accurately describe that...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


