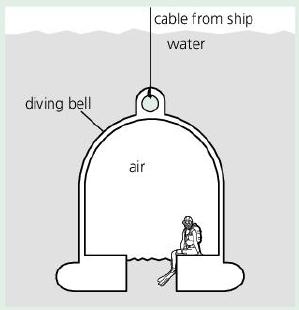
The diving bell below contains trapped air at the same pressure as the water outside. At the
Question:
The diving bell below contains trapped air at the same pressure as the water outside. At the surface, air pressure is 100 kPa. As the bell descends, the pressure on it increases by 100 kPa for every 10 m of depth.
a. What is the pressure on the diver at depths of 0 m, 10m, 20m and 30m?
b. At the surface, the bell holds 6 m3 of air. If the bell is lowered to a depth of 20 m, and no more air is pumped into it, what will be the volume of the trapped air? (Assume no change in temperature.)
cable from ship water diving bell air
Step by Step Answer:
aPressure at 0m depth no water only air pressureSo pressure is 100k...View the full answer



Related Video
For this experiment we need-One glass bottle (opening slightly smaller than an egg)-A matchbox-One peeled boiled eggIn this demonstration, the lit matchsticks heat the air inside the bottle. When air is heated it expands and some of it escapes out of the bottle. When the matches go out, the air inside the bottle cools and contracts thus creating a lower air pressure area inside the bottle than outside. Normally, the high-pressure air outside the bottle would come rushing in to equalize the low-pressure air in the bottle. The problem is that the egg is in the way. The air molecules on the outside of the bottle push the egg into the bottle.•Procedure• Place a glass bottle on the table.• Boil a normal-sized egg and• Place the boiled egg on the face or neck of the bottle.• We can see the egg cannot be pushed into the bottle because of air inside the bottle.• Now light the matches at the same time and drop them into the bottle.• Once the matchsticks have been dropped into the bottle Quickly Place the egg over the neck of the bottle.• You can see the egg is sucked into the bottle.This represents the air pressure and characteristics of air.
Students also viewed these Sciences questions
-
A 3 m3 tank initially contains air at 100 kPa and 25oC. The tank is connected to a supply line at 550 kPa and 25oC. The valve is opened, and air is allowed to enter the tank until the pressure in the...
-
A 10 m3 rigid tank contains air at 200 kPa and 150oC. A 1 kW internal heater is turned on. Determine the rate of change of (a) Stored energy (b) Temperature. (c) Pressure of air in the tank. Use the...
-
Air is pumped into and withdrawn from a 10 m3 rigid tank as shown in the accompanying figure. The inlet and exit conditions are as follow. Inlet: v1 = 2 m3/kg, V1 = 10 m/s, A1 = 0.01 m2; Exit: v2 = 5...
-
What are the energies of the two longest-wavelength lines in the Paschen series for hydrogen? What are the corresponding wavelengths? Give your answers to two significant figures.
-
An electric heater coil provided heat to a 15.5-g sample of iodine, I2, at the rate of 3.48 J/s. It took 4.54 min from the time the iodine began to melt until the iodine was completely melted. What...
-
You are a manager at Percolated Fiber, which is considering expanding its operations in synthetic fiber manufacturing. Your boss comes into your office, drops a consultant's report on your desk, and...
-
One area that fast-food restaurants compete with one another is with the service time for the drive-through window. The following data show the drive-through service times, in minutes, for a random...
-
How does professional identity influence what Jackie might do in this case?
-
The following data relate to the operations of Shilow Company, a wholesale distributor of consumer goods: Current assets as of March 3 1 : Cash $ 8 , 1 0 0 Accounts receivable $ 2 2 , 4 0 0 Inventory...
-
An exercise advocate wants to determine the effect that walking rigorously has on weight loss. The researcher recruits participants to engage in a weeklong study. The researcher instructs...
-
A car has a mass of 900 kg. It accelerates from rest at a rate of 1.2 m/s 2 . a. Calculate the time taken to reach a velocity of 30 m/s. b. Calculate the force required to accelerate the car at a...
-
The diagram below shows a uniform metre rule, weight W, pivoted at the 75 cm mark and balanced by a force of 2 N acting at the 95 cm mark. a. Calculate the moment of the 2 N force about the pivot. b....
-
Rowlock Ltd was incorporated on 1 October 2008 to acquire Rowlocks mail order business, with effect from 1 June 2008. The purchase consideration was agreed at 35,000 to be satisfied by the issue on 1...
-
8 Project two 15 UTSA Project two M Question 1 - Project two ChatGPT C chegg.com/homework-he X Course Hero how to take a sxreen shot X +...
-
Charitable purposes: Section 3(1) Charities Act 2011 1. Prevention or relief of poverty 2. Education 3. Religion, now includes: 4. - - A religion which involves belief in more than one god A religion...
-
Jack Price, The finance director of Humpty Doo Investment Ltd ( HDIL ) , is unsure whether he should consolidate some of the investments that the company owns. He has asked your advice as business...
-
Use QM to solve this problem. Suppose that Peter Cartman is deciding whether to invest in a bond mutual fund or a stock fund. Both bond and stock funds are sensitive to changing market conditions....
-
George Francis works at Gentry Medical Center which is in sunny Florida. The Medical Center experiences a higher volume of business closer to fall when many of the patients return for the winter from...
-
Youre trying to save to buy a new $175,000 Ferrari. You have $35,000 today that can be invested at your bank. The bank pays 2.9 percent annual interest on its accounts. How long will it be before you...
-
(a) Water flows through the nozzle of a garden hose. Find an expression for m in terms of line pressure P 1 , ambient pressure P 2 , inside hose diameter D 1 , and nozzle outlet diameter D 2 . Assume...
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (g) (h) SH
-
Paclitaxel (marketed under the trade name TaxolTM) is found in the bark of the Pacific yew tree, Taxus berevifolia, and is used in the treatment of cancer: (a) Draw the enantiomer of paclitaxel. (b)...
-
Predict the major product(s) for each of the following reactions: a. b. c. d. (PPH3)3RHCI H;o*
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...
-
Apple inc. CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (In milions, except number of shares which are reflected in thousands and par value) LABILITES AND SHAREHOLDERS' EQUITY: Current...

Study smarter with the SolutionInn App


