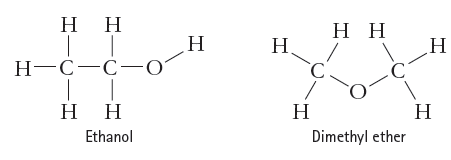
Account for the observation that ethanol, C 2 H 5 OH, dissolves readily in water, but dimethyl
Question:
Account for the observation that ethanol, C2H5OH, dissolves readily in water, but dimethyl ether, CH3OCH3,
which has the same number and kinds of atoms, does not.
Transcribed Image Text:
нн н Н. Н Н Н-С—С-О нн Ethanol Н Dimethyl ether Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (21 reviews)
The arrangement of atoms within a molecule makes all the differ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Physics questions
-
At 10C, which is more concentrated: a saturated solution of sodium nitrate, NaNO 3 , or a saturated solution of sodium chloride, NaCl? (See Figure 16.20 .) NaNO3 180 160 140 120 LicI 100 80 60 KCI 40...
-
Why can 500 mL of fresh water absorb more gaseous carbon dioxide than 500 mL of sugar water at the same temperature?
-
Is the decomposition of food by bacteria in our digestive systems aerobic or anaerobic? What evidence supports your answer? How do composting toilets work to remove the bad smells of human waste?
-
The following table describes a randomized trial comparing an experimental medication to a placebo for treatment of reflux. Experimental Treatment Placebo (n = 100) Patient Characteristics (n = 100)...
-
Fokine Research Institute has three departments: biology, chemistry, and physics. The institute's controller wants to estimate the cost of operating each department. He has identified several...
-
What are some of the tasks at which robots excel? Which human tasks are difficult for them to master? What fields of AI are required to develop a truly perceptive robot?
-
Define decision making.(p. 208)
-
The average stock price for companies making up the S&P 500 is $30, and the standard deviation is $8.20 (BusinessWeek, Special Annual Issue, Spring 2003). Assume the stock prices are normally...
-
The Angelina Corporations common stock has a beta of 1.4. If the risk-free rate is 4.8 percent and the expected return on the market is 12 percent, what is the companys cost of equity capital? (Do...
-
Big CPA Firm has many partners in one of its local offices. Two of these partners are Tom, a tax partner, and Alice, an audit partner. Because of the size of the office, Tom and Alice do not know...
-
The air a scuba diver breathes is pressurized to counteract the pressure exerted by the water surrounding the divers body. Breathing the high-pressure air causes excessive amounts of nitrogen to...
-
Why is rain or snow called precipitation?
-
A rope, under a tension of 200 N and fixed at both ends, oscillates in a second-harmonic standing wave pattern. The displacement of the rope is given by y = (0.10 m) (sin x/2) sin 12t, where x = 0 at...
-
A farmer has an acre of specialty vegetables and is preparing for the summer harvest. Historically, this acre has yielded an average of 2,100 lbs of product with a standard deviation of 950 lbs. A...
-
Solve 3x 82+22 = (4).
-
(c) Compute EVPI and EVSI (in thousands of dollars). (Round your answers to one decimal place.) EVPI $ 3.6 EVSI $ 3.6 Xthousand x thousand Discuss whether the firm should consider a consulting expert...
-
Question 9 (1 point) If the common law requires employees of a bar establishment to monitor a potentially intoxicated patron and to possibly make an effort to intervene if there is an indication the...
-
B. A velocity potential is given by the equation: Q = x-y 3. (10 pts) Short answer, What special characteristics of the velocity potential make it very useful in identifying a type of flow and...
-
Find the remaining trigonometric ratios. cot B = 3, < B < 2
-
Discuss whether responsible human resources management should apply different standards for the home company and suppliers, for developed countries and developing countries, and for large companies...
-
The jet pump injects water at V 1 = 40 m/s through a 7.6 cm pipe and entrains a secondary flow of water V 2 = 3 m/s in the annular region around the small pipe. The two flows become fully mixed...
-
Water flows steadily through the piping junction, entering section 1 at 0.0013 m 3 /s. The average velocity at section 2 is 2.1 m/s. A portion of the flow is diverted through the shower head, which...
-
The V-shaped tank has width w into the paper and is filled from the inlet pipe at volume flow Q. Derive expressions for (a) the rate of change dh/dt and (b) the time required for the surface to rise...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


