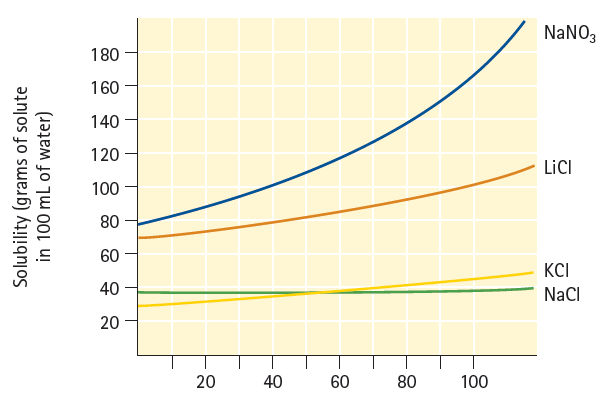
At 10C, which is more concentrated: a saturated solution of sodium nitrate, NaNO 3 , or a
Question:
At 10°C, which is more concentrated: a saturated solution of sodium nitrate, NaNO3, or a saturated solution of sodium chloride, NaCl? (See Figure 16.20.)
Transcribed Image Text:
NaNO3 180 160 140 120 LicI 100 80 60 KCI 40 NaCI 20 20 40 80 60 100 Solubility (grams of solute in 100 ml of water)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
At 10C a saturated solut...View the full answer

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
The solubility of sodium bicarbonate in water is 11.1 g NaHCO 3 /l0O g H 2 O at 30C and 16.4 g NaHCO 3 /100 g H 2 0 at 60C. If a saturated solution of NaHCO 3 at 60C is cooled and comes to...
-
Consider a beaker containing a saturated solution of CaF2 in equilibrium with undissolved CaF2 (s). (a) If solid CaCl2 is added to this solution, will the amount of solid CaF2 at the bottom of the...
-
Consider a beaker containing a saturated solution of Pbl2 in equilibrium with undissolved Pbl2(s). (a) If solid KI is added to this solution, will the amount of solid PbI2 at the bottom of the beaker...
-
In Figure, a square of edge length 20.0 cm is formed by four spheres of masses m1 = 5.00 g, m2 = 3.00 g, m3 = 1.00 g, and m4 = 5.00 g. In unit-vector notation, what is the net gravitational force...
-
Lamb Tools Company has two production departments in its manufacturing facilities. Home tools specializes in hand tools for individual home users, and professional tools makes sophisticated tools for...
-
Imagine that you are developing the rules for an expert system to select the strongest candidates for a medical school. What rules or heuristics would you include?
-
A Final Word on Decision Making and Critical Thinking (p. 208)
-
Grinder Ltd. is an S corporation that is wholly owned by Juan Plowright. Because several of Juan's ancestors have had Alzheimer's disease, Juan is transferring many of his assets to trusts, and he is...
-
Toxaway Company is a merchandiser with two divisions Commercial and Residential. The company s accounting intern was asked to prepare segmented income statements the company s divisional managers...
-
1. Would you characterize television programming decisions as structured or unstructured? Explain. What type of decision-making condition would you consider this to be? Explain. 2. What criteria did...
-
Account for the observation that ethanol, C 2 H 5 OH, dissolves readily in water, but dimethyl ether, CH 3 OCH 3 , which has the same number and kinds of atoms, does not. . -- Ethanol Dimethyl...
-
Why is rain or snow called precipitation?
-
Gilroy, Sims & Associates, Ltd., was a limited partnership engaged in real estate development. The original general partners were Richard Gilroy and William Sims. Thomas Green and John Murphy, Jr.,...
-
Ted sold his Microsoft stock for $40,000 paying a commission of $800. He purchased the stock in 2004 for $8,000 and paid commission of $200. What is the recognized gain on the sale?
-
Liquid water at 80C and at 1atm flows through a heated pipe at a flow rate of 3.1 kg/s. It then leaves the pipe as steam. The water receives 9753840 J of heating from the pipe. Calculate the...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Find the value of the sum.
-
Briefly describe the following types of group life insurance plans: a. Group term life insurance b. Group accidental death and dismemberment insurance (AD&D) c. Group universal life insurance d....
-
A thin layer of liquid, draining from an inclined plane, has a velocity profile v x v 0 (2y/h - y 2 /h 2 ), where v 0 is the surface velocity. If the plane has width 10 cm into the paper, determine...
-
Two very long parallel plates of length 2L are separated a distance b. The upper plate moves downward at a constant rate V. A fluid fills the space between the plates. Fluid is squeezed out between...
-
In the figure below, the x-direction velocity profiles are shown for a control volume surrounding a cylinder. If the flow is in-compressible, what must the rate of flow be across the horizontal...
-
Transcribed image text
-
QUESTION 20 Assume a company reported the following results Sales Net operating income Average operating assets Margin Turnover Return on investment (ROI) 5300,000 2 $240.000 40% ? 2 What is the net...
-
2. Using the graph provided below, determine the fixed cost, the total variable cost, the variable cost per unit, and the TOTAL COST to produce 60 units. Fixed Cost ______________ Variable Cost...

Study smarter with the SolutionInn App


