Question: Explain why, for these three substances, the solubility in 20C water goes down as the molecules get larger, but the boiling point goes up. Boiling
Explain why, for these three substances, the solubility in 20°C water goes down as the molecules get larger, but the boiling point goes up.
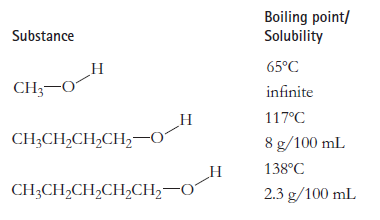
Boiling point/ Solubility Substance 65C CH;-0 infinite 117C CH;CH,CH,CH,0 8 g/100 mL 138C CH3CH,CH,CH,CH20 2.3 g/100 mL
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
The boiling points go up because of an increase in the number of molecular interactions between mol... View full answer

Get step-by-step solutions from verified subject matter experts


