A sample Mg metal reacts with excess aqueous hydrochloric acid to produce 257.3 mL of dry hydrogen at a pressure of 1.00 atm and
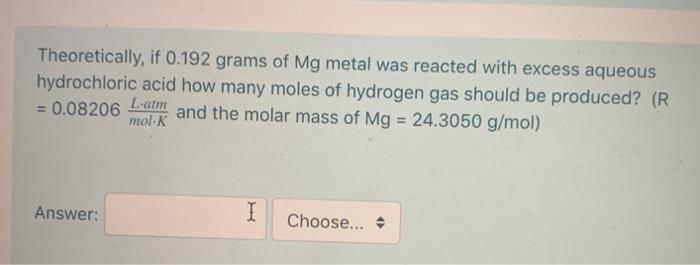
A sample Mg metal reacts with excess aqueous hydrochloric acid to produce 257.3 mL of dry hydrogen at a pressure of 1.00 atm and a temperature of 27.1 C. How many moles of hydrogen gas are produced? (R = 0.08206 Lam :) mol-K Answer: Theoretically, if 0.192 grams of Mg metal was reacted with excess aqueous hydrochloric acid how many moles of hydrogen gas should be produced? (R = 0.08206 and the molar mass of Mg = 24.3050 g/mol) L-atm mol-K Answer: I Choose...
Step by Step Solution
3.43 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
moles of Hy 2 volum Pressure x gas con...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e424c946dd_183376.pdf
180 KBs PDF File
635e424c946dd_183376.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started



