Question: How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it
How readily an acid donates a hydrogen ion is a function of how well the acid is able to accommodate the resulting negative charge it gains after donating. Which should be the stronger acid: water or hypochlorous acid? Explain.
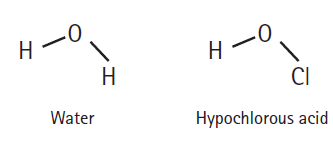
0- H- 0- CI Hypochlorous acid Water
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
These molecules behave as acids by losing the hydrogen ion from the oxygen atom which takes o... View full answer

Get step-by-step solutions from verified subject matter experts


