Question: Which should be the stronger base: ammonia, NH 3 , or nitrogen trifluoride, NF 3 ? F- N-F H - N-H Ammonia Nitrogen trifluoride
Which should be the stronger base: ammonia, NH3, or nitrogen trifluoride, NF3?
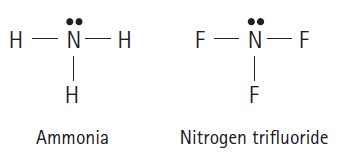
F- N-F H - N-H Ammonia Nitrogen trifluoride
Step by Step Solution
3.33 Rating (171 Votes )
There are 3 Steps involved in it
To answer this question ask yourself which base might be more willing to ... View full answer

Get step-by-step solutions from verified subject matter experts


