Question: If you saw the label phenylephrine . HCl on a decongestant, would you worry that consuming it would expose you to the strong acid hydrochloric
If you saw the label phenylephrine . HCl on a decongestant, would you worry that consuming it would expose you to the strong acid hydrochloric acid? Explain.
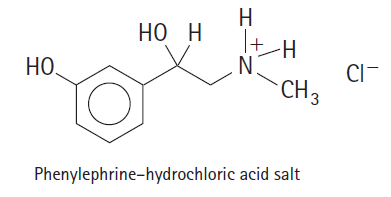
It H . N. CI- CH3 Phenylephrine-hydrochloric acid salt
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
No This label indicates that it contains the hydrogen chloride ... View full answer

Get step-by-step solutions from verified subject matter experts


