Rank the following molecules from least oxidized to most oxidized: Ethane Ethanol ||
Question:
Rank the following molecules from least oxidized to most oxidized:
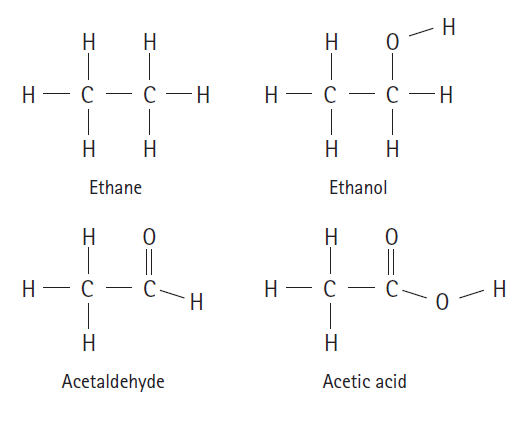
Transcribed Image Text:
Н Н Н Н — с — с —Н Н— с — с —Н Н Н Ethane Ethanol Н Н || Н — с- Н — с — С—н Н Н Н Acetaldehyde Acetic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
From left to right these compounds are presented in order of least oxidized ...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Physics questions
-
Rank the following molecules in order of increasing boiling point (without looking up the real values!): (a) 3-methylheptane; (b) Octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.
-
Rank the following molecules in order of the phase they form at room temperature: solid, liquid, gas. a. b. CH 3 CH 2 CH 2 CH 3 c. CH 3 CH 2 CH 2 CH 2 - OH CH H;C- C-
-
Rank the mass of these molecules from most to least. H. H. (. B A
-
Based on the information in Problems 9 and 10, what is Ryan and Nicoles liquidity ratio? What is their debt to asset ratio? Comment on each ratio. In Problems 10 Mortgage......... $ 43,500 Car...
-
Walter, who is single, owns a personal residence in the city. He also owns a cabin near a ski resort in the mountains. He uses the cabin as a vacation home. In August, Walter borrowed $60,000 on a...
-
If the force applied to the handle of the load binder is 50 lb, determine the tensions T 1 and T 2 in each end of the chain and then draw the shear and moment diagrams for the arm ABC. 50 lb F12 in.-...
-
2. How much was income from Vasquez in 2013?
-
Condensed financial data of Minnie Hooper Company are shown below. Additional information:1. New plant assets costing $146,000 were purchased for cash during the year.2. Investments were sold at...
-
Rows below show the monthly closing prices of three stocks between 2012 and 2018. The data were collected from Yahoo Finance. When answering these questions, ignore dividends and assume all prices...
-
As of January 1, 2012, the trial balance for Haven Hospital was as follows: During the fiscal year ended December 31, 2012, the following transactions occurred: 1. Patient service revenue amounted to...
-
An acid and a base react to form a salt, which consists of positive and negative ions. Which forms the positive ions: the acid or the base? Which forms the negative ions?
-
Review the concept of electronegativity in Section 15.6, and rank these elements from the weakest to strongest reducing agent: (a) chlorine, Cl; (b) sulfur, S; (c) sodium, Na.
-
What will be the impact (increase or decrease) of the following transactions on a companys cash in its cash flow statement? (a) The company pays its suppliers early. (b) Inventory is purchased in...
-
Share your thoughts on the descriptions of coaching versus mentoring. Discuss which technique you personally find more helpful, incorporating your peers' example scenarios if possible. Provide...
-
Hanung Corp has two service departments, Maintenance and Personnel. Maintenance Department costs of $380,000 are allocated on the basis of budgeted maintenance-hours. Personnel Department costs of...
-
Discuss difference between nominal interest rate and real interest rate. Explain why real interest rate is more important than the nominal interest rate using your answer to Question 1 of the...
-
Refer to Figure 14-1. How would an increase in the money supply move the economy in the short and long run?
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
Which is denser, the granitic rock of the continents or the basaltic rock of the ocean floors? Which extends deeper into the crust, the continents or the ocean floors?
-
The Zwatch Company manufactures trendy, high-quality moderately priced watches. As Zwatch's senior financial analyst, you are asked to recommend a method of inventory costing. The CFO will use your...
-
For the pump having the characteristics shown in Problem 14.14, operating at maximum efficiency with the speed increased to 1000 rpm, what will be (a) the new discharge flow rate and (b) the power...
-
The pump having the characteristics shown in Problem 14.14 is to be operated at 800 rpm. What discharge rate is to be expected if the head developed is 410 m? Data From Problem 14.14 Performance...
-
If the pump having the characteristics shown in Problem 14.14 is tripled in size but halved in rotational speed,what will be the discharge rate and head when operating at maximum efficiency? Data...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


